Halia bara Rhizome
Zingiber officinale Roscoe var. rubrum Theilade
Zingiberaceae
DEFINITION
Halia bara rhizome consists of the dried rhizome of Zingiber officinale Roscoe var. rubrum Theilade (Zingiberaceace).
SYNONYM
Amomum zingiber L [ 1 ].
VERNACULAR NAMES
Ginger (English); halia udang, halia merah, halia bara (Malay) [ 2 ].
CHARACTER
| Colour | Yellowish brown colour |
| Odour | Aromatic characteristic |
| Taste | Pungent aromatic taste |
IDENTIFICATION
Plant Morphology
Z. officinale var. rubrum is a slender perennial plant. Stem are dark green with a prominent midrib sheathed at the base with 30-60 cm in height; the second and later stems are shorter and terminate in flat bracts with few ancillary flowers. Leaves green in colour, narrow, 25-30 cm in length, 4-6 cm in width. Flower yellowish red in colour and are sparse. Rhizome red on the outside with a yellow to pinkish cross-section, base of its leaf shoot is red, branched, arranged laterally under the soil surface [ 2 ].
Microscopy
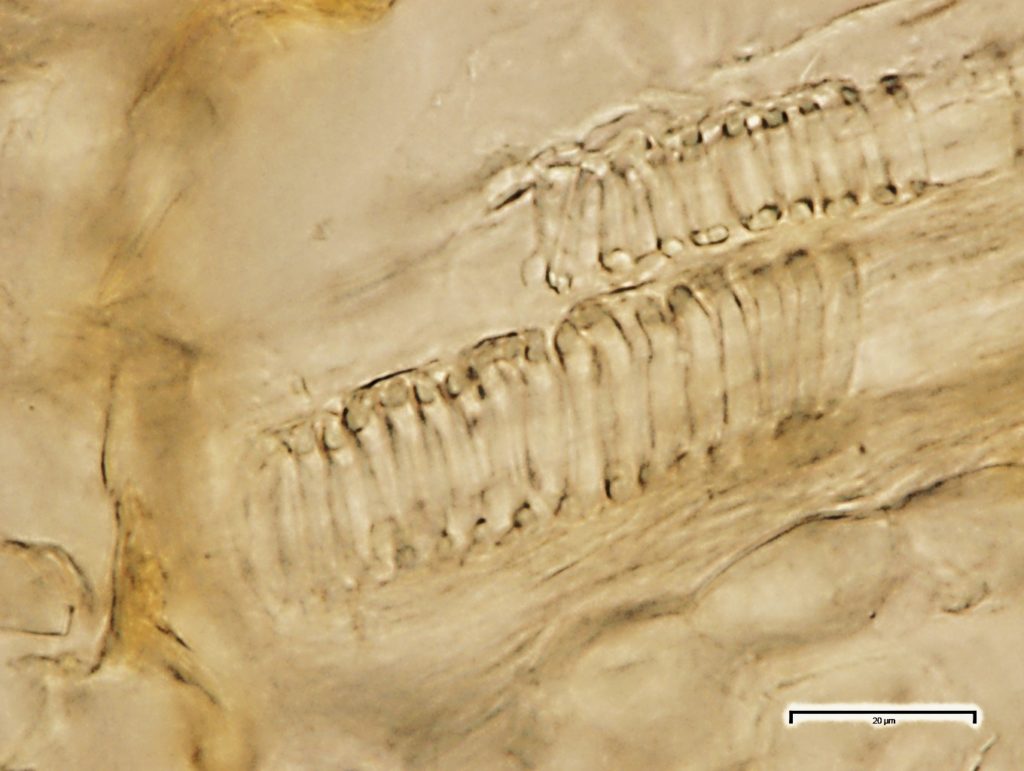
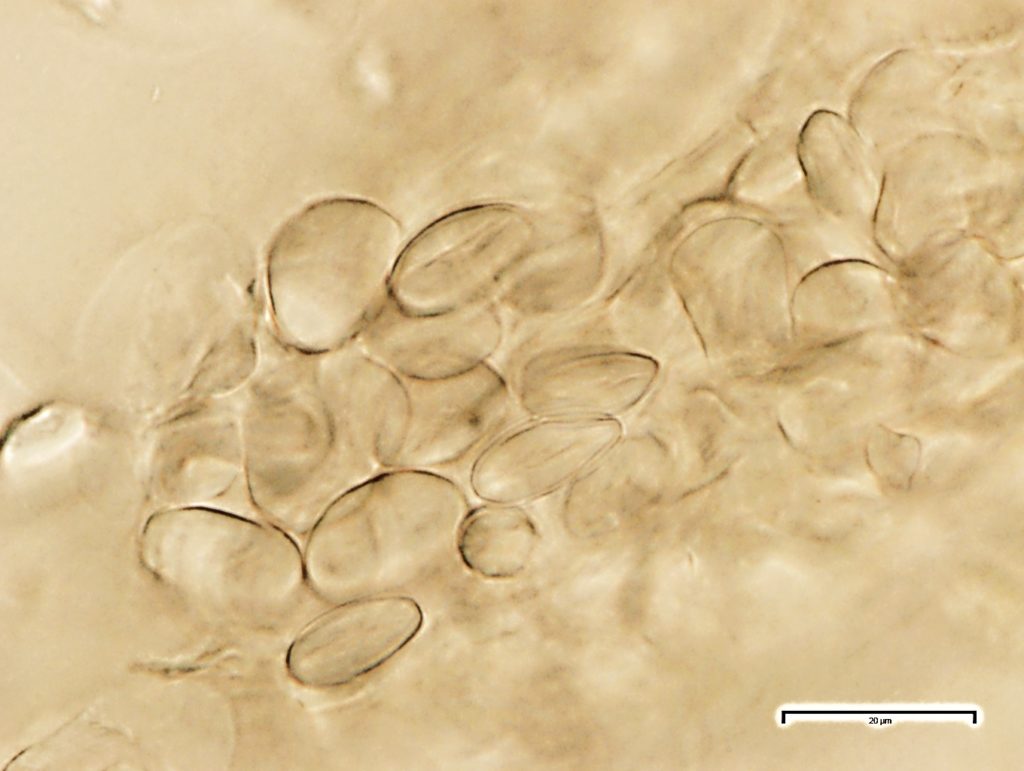
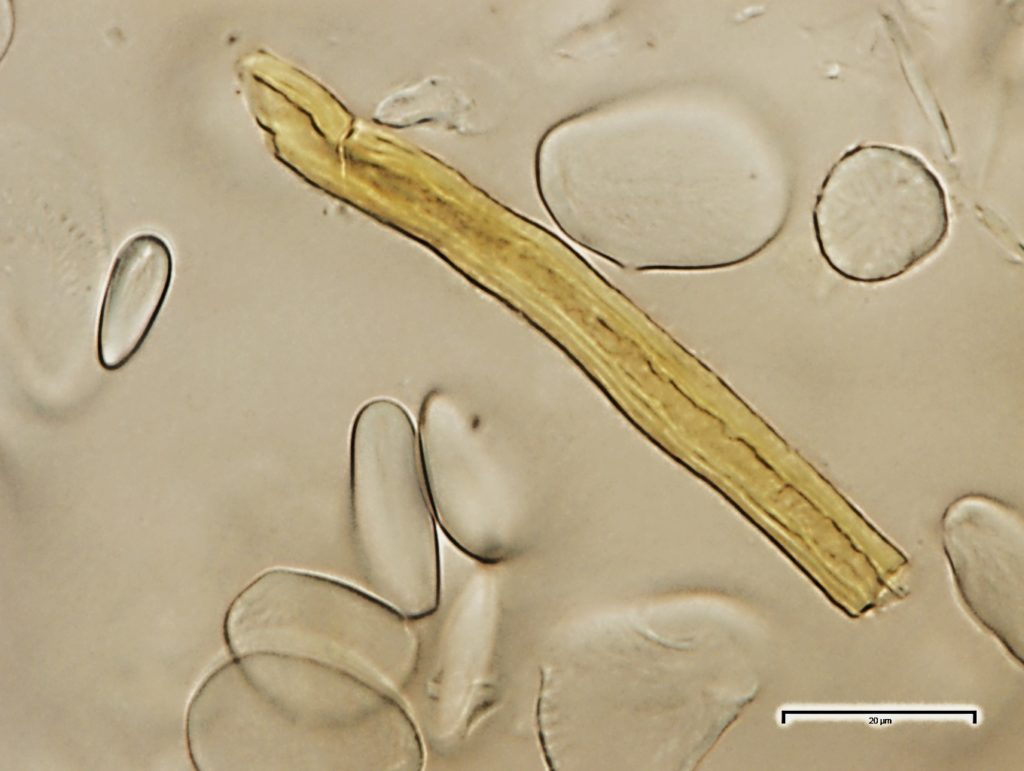
The abundant starch granules are almost all simple, of varying sizes from round to oval, oblong to sub rectangular in outline; compound granules with two components occur very rarely; the fibres are found in groups, sometimes found associated with the vessels, with thin dentate wall; the vessels are fairly large and scarce, usually found in groups, reticulate thickened frequently showing regularly arranged rectangular pits, spirally thickened vessels are much smaller and very few.
Colour Tests
Observed colour of solution after treatment with various reagents:
| HCl (conc.) | Brown |
| 5% NaOH | Brown |
| 25% NH4OH | Brown |
Thin Layer Chromatography (TLC)
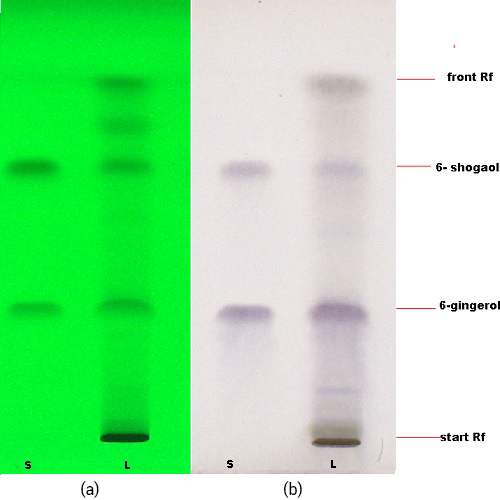
Figure 3 : TLC profiling of a mixture of 6-gingerol and 6-shogaol standards (S) and methanol extract of Z. officinale var. rubrum (L) at (a) 255 nm; (b) after spraying with anisaldehyde-sulphuric acid reagent.
| Test Solutions | Weight about 1.0 g of Z. officinale var. rubrum dried rhizome powder in a 50 mL flat bottom flask. Add 10 mL ethanol. Reflux the mixture for 35 min at 80°C. Cool the mixture and allow the insoluble matter to settle. Filter the mixture and use the filtrate as the test solution. |
| Standard solution | Separately dissolve 6-gingerol [CAS no.:23513-14-6] and 6-shogaol [CAS no.: 555-66-8] in methanol. Mix the standard solutions to produce 6-gingerol 0.5 mg/mL and 6-shogaol 0.4 mg/mL. |
| Stationary Phase | Glass plate HPTLC Si 60 F254 5×10 cm, Merck |
| Mobile phase | Toluene: ethyl acetate (7.5:2.5) (v/v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
| Detection |
|
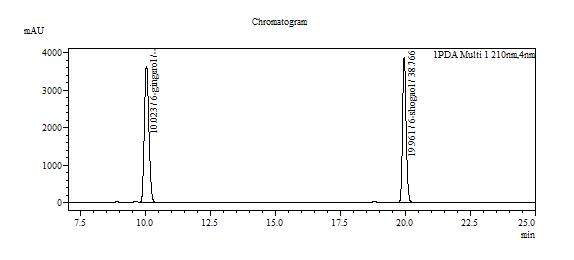
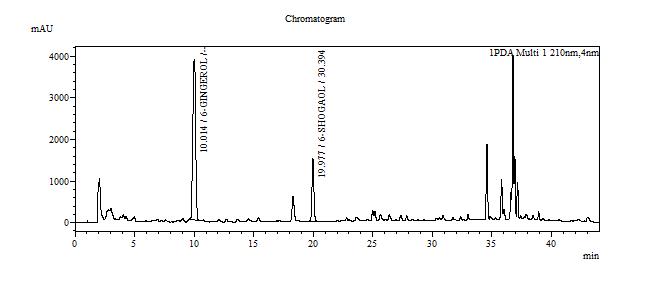
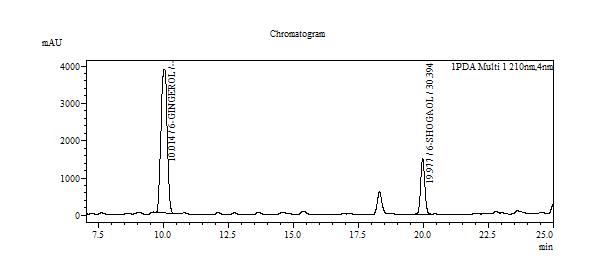
High Performance Liquid Chromatography (HPLC)
| Test solution | Extract about 2.0 g of Z. officinale var. rubrum dried rhizome powder with 20 mL ethanol in a 250 mL round bottom flask. Reflux the mixture for 35 minutes at 80°C. Filter the mixture solution through a 0.45 µm syringe filter and inject the filtrate into the HPLC column. | ||||||||||||||||||||||||
| Standard solution | Separately dissolve 6-gingerol [CAS no.:23513-14-6] and 6-shogaol [CAS no.: 555-66-8] in methanol. Mix the standard solutions to produce 6-gingerol 0.5 mg/mL and 6-shogaol 0.4 mg/mL. | ||||||||||||||||||||||||
| Chromatographic system |
Detector: UV 210 nm Column: C18 column (250 × 4.6 mm I.D, 5.0 µm particle size) Column oven temperature: 48°C Flow rate: 1.0 mL/min Injection volume: 20 µL |
||||||||||||||||||||||||
| Mobile Phase (Isocratic mode) |
|
||||||||||||||||||||||||
| System suitability requirement |
Perform at least five replicate injections of the standard solutions (0.5 mg/mL). The requirements of the system suitability parameters are as follow:
|
||||||||||||||||||||||||
| Acceptance criteria |
|
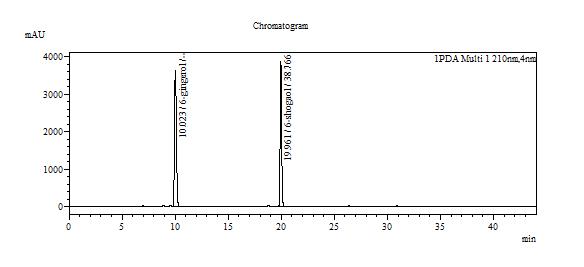
Table 1 : The Relative Retention Time (RRT) for the four characteristic peaks
| Standard | RRT |
| 6-gingerol (as reference) | 1.00 |
| 6-shogaol | 1.99 |
Note: The RRTs provided only serve as a guidance
PURITY TESTS
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 8% |
| Acid-insoluble ash | Not more than 2% |
| Loss on Drying |
| Not more than 14% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 22% |
| Cold method | Not less than 17% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 9% |
| Cold method | Not less than 6% |
SAFETY TESTS
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Methanol extract of Z. officinale var. rubrum dried rhizome has been reported to contain 6-gingerols and 6-shogaols [ 3 ].
Ethanol extract of Z. officinale var. rubrum dried rhizome of has been reported to contain amino acids (e.g. glutamine, glutamic acid, threonine, leucine, lysine, valine and tyrosine)
[ 4 ].
Methanol extract of Z. officinale var. rubrum dried rhizome has been reported to contain flavonoids (e.g. quercetin, rutin, catechin, epicatechin and kaempferol) [ 5 ].
Phosphoric acid extract of Z. officinale var. rubrum dried rhizome of has been reported to contain phenolics (e.g. gallic acid and ferulic acid) and cyano group (cyanide) [ 5 , 6 ].
Essential oil of the Z. officinale var. rubrum rhizome has been reported to contain monoterpenoids (e.g. camphene, geranyl acetate, geranial, neral, geraniol, 1,8-cineole [ 7 ] and oxygenated monoterpenes (e.g. E-citral, β-citronellol, trans-geraniol and borneol) [ 8 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
Information and data have not been established.
Biological and pharmacological activities supported by experimental data
Antimicrobial activity
Essential oil of Z. officinale var. rubrum rhizome (2.5 mg/mL) inhibited the growth of Bacillus licheniformis with minimum inhibition concentration (MIC) of 0.16 mg/mL, Bacillus spizizenii (MIC = 0.24 mg/mL), Staphylococcus aureus (MIC = 0.31 mg/mL), Escherichia coli (MIC = 0.31 mg/mL), Klebsiella pneumonia (MIC = 0.47 mg/mL) and Pseudomonas stutzeri (MIC = 0.63 mg/mL) compared to tetracycline (B. licheniformis = 0.001 mg/mL, B. spizizenii = 0.0018 mg/mL, S. aureus = 0.0077 mg/mL, E. coli = 0.0156 mg/mL, K. pneumonia = 0.0037 mg/mL, P. stutzeri = 0.0081 mg/mL) using micro-dilution assay [ 8 ].
Ethyl acetate extract of Z. officinale var. rubrum (50 mg/mL) inhibited the growth of Bacillus subtilis with inhibition zones of 7.5 mm compared to gentamycin (19.0 mm) and ampicilin (38.5 mm) using agar disc diffusion assay [ 9 ].
Anti-oxidant activity
Methanol extract of Z. officinale var. rubrum (10–50 µg/mL) showed anti-oxidant activity with 1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenging activity (inhibition concentration at 50% of growth (IC50) of 28.2 µg/mL) compared to α-tocopherol (IC50 = 16.2 µg/mL) and butylated hydroxytoluene (IC50 = 20.4 µg/mL) using DPPH assay [ 10 ].
Methanol extract of Z. officinale var. rubrum (100 mg/mL) showed anti-oxidant activity with ion iron(II) reducing ability (770.4 – 788.57 µmol Fe(II)/g) compared to α-tocopherol (953 µmol Fe(II)/g) using ferric reducing anti-oxidant potential. The total flavanoid content was found to be 3.97–4.34 mg quercetin/g while total phenolic content was 9.48–11.53 mg gallic acid/g [ 11 ].
Methanol extract of Z. officinale var. rubrum rhizome (1–200 µg/mL) showed anti-oxidant activity with inhibition of lipid peroxidation (78.73%) compared to quercetin (94.7%) using thiobarbituric acid (TBA) assay [ 12 ].
Antitumour activity
Methanol extract of Z. officinale var. rubrum rhizome (4.69–300 μg/mL) showed anti-tumour activity against breast cancer cell lines MCF-7 with inhibition concentration at 50% of growth (IC50) of 46.8 μg/mL and breast cancer cell lines MDA–MB–231 (IC50 = 39.2 μg/mL) compared to tamoxifen (IC50 = 19.1 and 22.61 μg/mL, respectively) [ 12 ].
Cytotoxic activity
Methanol extract of Z. officinale var. rubrum rhizome (37.5 μg/mL) showed 92.38% cell viability of mammary epithelial cells MCF-10A compared to tamoxifen (0% cell viability)
[ 12 ].
Ethanol (50%) extract of Z. officinale var. rubrum rhizome (200, 400 and 600 mg/kg) administered orally to cigarette smoke-induced male Sprague Dawley rats (4 – 6 months old) for duration of 21 days significantly (p < 0.05) increased sperm count (3.36–5.56 million/mL), percentage of motility (18.0 – 26.4%) and morphology (63.2–75.1%) compared to control (sperm count = 4.76 million/mL, percentage of motility = 14.2%, percentage of morphology = 55.0%) [ 13 ].
Clinical studies
A clinical trial to study the antiemetic effect of Z. officinale var. rubrum on chemotherapy-induced nausea and vomiting was conducted involving 20 cancer patients who were receiving chemotherapy CAF (cyclophosphamide, adriamycin-5-fluorouracil) and antiemetic drugs (ondasentron and dexamethasone). These patients were divided into two groups namely a group given antiemetic drugs and a group given antiemetic drugs concurrently with infusion of Z. officinale var. rubrum (10 g) thrice daily for five days from second day after chemotherapy. Value of nausea and vomiting measured seven times significantly (p < 0.05) decrease in the treatment group receiving infusion. The incidence of nausea and vomiting also showed significant (p < 0.05) difference compared to control group [ 15 ].
SAFETY INFORMATION
Preclinical studies (Toxicological studies)
Acute toxicity
Ethanol (95%) extract of Z. oficinale var. rubrum rhizome from administrated orally (2–5 g/kg) as a single dose to male and female Sprague Dawley rats (150-180 g body weight) for 14 days. No toxicity effect was observed in treated rats (LD50 value > 5 g/kg) [ 14 ].
Oral single dose acute toxicity study on female Sprague Dawley rats ( aged between 8 and 12 weeks old) using aqueous extract of E. elatior flowers showed no toxic effect on the parameters observed, including behaviors, body weight , food and water intake. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. No observed -adverse-effect level (NOAEL) is more than 2,000 mg/kg body weight [ 16 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- The plant list. [Internet] Zingiber officinale var. rubrum. [cited on 23rd December 2014]. Available from: http://www.theplantlist.org/tpl1.1/record/kew-219561
- Musa Y, Azimah K, Zaharah H. Tumbuhan ubatan popular Malaysia. Serdang: MARDI. 2009;p.44.
- Parthasarathy VA, Chempakam B, Zachariah TJ. Chemistry of spices. London,UK, CABI; 2008: 70-97.
- Ghasemzadeh A, Jaafar HZ, Karimi E, Ashkani S. Changes in nutritional metabolites of young ginger (Zingiber officinale Roscoe) in response to elevated carbon dioxide. Molecules. 2014;19(10):16693-706.
- Ghasemzadeh A, Jaafar HZ, Rahmat A. Identification and concentration of some flavonoid components in Malaysian young ginger (Zingiber officinale Roscoe) varieties by a high performance liquid chromatography method. Molecules. 2010;15(9):6231-6243.
- Ghasemzadeh A, Jaafar HZ, Rahmat A. Elevated carbon dioxide increases contents of flavonoids and phenolic compounds, and antioxidant activities in Malaysian young ginger (Zingiber officinale Roscoe.) varieties. Molecules. 2010;15(11):7907-7922.
- Sukari MA, Mohd Sharif NW, Yap ALC, Tang SW, Neoh BK, Rahmani M, Ee GCL, Yusof UK. Chemical constituents variations of essential oils from rhizomes of four Zingiberaceae species. 2008;12(3):638-644.
- Sivasothy Y, Chong WK, Hamid A, Eldeen IM, Sulaiman SF, Awang K. Essential oils of Zingiber officinale var. rubrum Theilade and their antibacterial activities. Food chemistry. 2011;124(2):514-517.
- Philip K, Malek SN, Sani W, Shin SK, Kumar S, Lai HS, Serm LG, Rahman SNSA. Antimicrobial activity of some medicinal plants from Malaysia. American Journal of Applied Sciences. 2009;6(8):1613-1617.
- Ghasemzadeh A, Jaafar HZ, Karimi E, Ibrahim MH. Combined effect of CO2 enrichment and foliar application of salicylic acid on the production and antioxidant activities of anthocyanin, flavonoids and isoflavonoids from ginger. BMC Complementary and Alternative Medicine. 2012;12(1):229.
- Ghasemzadeh A, Jaafar HZ, Rahmat A, Wahab PEM, Halim MRA. Effect of different light intensities on total phenolics and flavonoids synthesis and anti-oxidant activities in young ginger varieties (Zingiber officinale Roscoe). International Journal of Molecular Sciences. 2010;11(10):3885-3897.
- Ghasemzadeh A, Jaafar HZ. Antioxidant potential and anticancer activity of young ginger (Zingiber officinale Roscoe) grown under different CO2 concentration. Journal of Medicinal Plants Research. 2011;5(14):3247-3255.
- Fitriana R, Susantiningsih T. Pengaruh pemberian ekstrak etanol jahe merah (Zingiber officinale Roxb var. rubrum) terhadap motilitas dan morfologi spermatozoa tikus putih (Rattus norvegicus) jantan strain Sprague Dawley yang dipapar asap rokok. Majority. 2011;8(3):128-164.
- Abdulaziz Bardi D, Halabi MF, Abdullah NA, Rouhollahi E, Hajrezaie M, Abdulla MA. In vivo evaluation of ethanolic extract of Zingiber officinale rhizomes for its protective effect against liver cirrhosis. BioMed Research International. 2013;2013:918460 (10 pages).
- Muthia R, Wahyu W, Dachriyanus. Effect of ginger infusion on chemotherapy induced nausea and vomiting in breast cancer patients. Journal of Biology, Agriculture and Healthcare. 2013;13(3):42-46.
- Syed Muhammad Asyraf ST, Ezarul Faradiana L, Nor Kasmini S, Wan Mohammad Adham Afiq WZ, Siau TC, Teh BP. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2016.Report no.: HMRC 11-045/01/ZOR/RH/B.