INTRODUCTION
The word ecology defined as the scientific study of the interactions between organisms and their environment and is derived from the Greek oikos, meaning home, and logos is knowledge. A scientist studies ecology in an ecosystem. Ecosystem is a space/area on planet earth where there are living organisms interacting among themselves and also with non-living components present. Ecosystem is used to denote the biological community together with the abiotic environment in which it is set. In this space/area one could trace the flow of energy from one organism to another, as well as see cycling of materials between living and non-living parts [1]. In layman’s term, ecosystem is a space which contains various living and non-living things. Interactions between the living things as well as between living and non-living components of environment resulted in transfer of energy from the various groups. A simple example, insects feed on plants, and frogs feed on insects. Larger predator like snakes will feed on frogs. This food chain shows the energy flowing from plants to insects to frogs to snakes. In turn, when the snake dies, decomposition takes place producing elements like Calcium, Natrium/Sodium, Potassium and compounds like Phosphate and so forth, which are nutrients for plants. These nutrients return to the soil to be taken up by plants to grow. This show a cycle between living components of the ecosystem (snake) and non-living components (nutrients). These interactions happen naturally in an ecosystem.
As defined in the Convention on Biological Diversity [2] document, biodiversity is at the level of genetic, species as well as ecosystem. The lowest level of biodiversity is the genetic biodiversity; where variations lie at the genetic level. The different genetic composition and make-up of individuals of the same species of organism may result in different size, shape, colour, texture, habit, behaviour of the individuals. In Fig. 1 are several examples of intra-specific variation. The big corn is relatively larger than the small corn. The big corn is well suited for eating the seed, either as corn on the cob, or seeds separated out from the cob and made into flour. The small corn is consumed as vegetable. They are called cultivars. Once upon a time, the wild varieties were selected by farmers to cultivate in their farms for domestic use, and since these varieties were modified and maintained as artificial populations by the farmers, they are called cultivars. Of course today many of the cultivars had made their ways into the commercial agriculture as commodity crops. Similarly, water melon in Malaysia comes in two colours – red and yellow, cultivars of the same species.
Of course, the easiest diversity that could be understood by a lay person is the species diversity. In two previous articles, we see the different number of species of plant and animals. Thus, between the various animals species a person could differentiate diversity easily, as in Fig. 2. A cow is different from a hawk and different form a snake. However, within the cow species, a hawk species and a snake species, there are variations that we call intraspecific variations which are attributed to environmental and genetic factors.

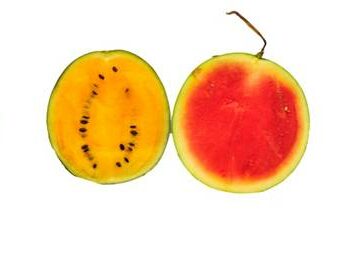
Fig. 1: Size variation may occur as in corn; colour variation in water melon.



Fig. 2: Different species are easily distinguishable by their external characteristics as seen in this photographs.
The highest level of diversity is the ecosystem diversity. At the ecosystem level it involves interactions between organisms and its biological and non-biological natural environment. In specific ecosystem, which is much determined by the dominant, naturally occurring vegetation, only certain animals could survive. Interactions between these peculiar regimes of plant and animal diversities are manifested in a specific ecosystem. Ecosystem diversity is much determined by basically the dominating vegetation types. Types of vegetation is in turn determined by the amount of sunlight an area received, soil type and geological formation. There are many different ecosystems found in the world. The most obvious are the marine and the terrestrial ecosystems. In this article, the marine ecosystem of Malaysia will be described first followed by the terrestrial ecosystems. In the terrestrial ecosystems, the aquatic freshwater ecosystems, such as peat swamps will be described followed by mangrove, an ecosystem that interfaces between the saline marine environment and the freshwater rivers of the inland. Thereafter, the dry forest will be described initially with a description of a typical tropical forest, then focusing down to the lowland tropical forest, hill, sub-montane and montane forests of Malaysia. Several other forest types such as beach forest, limestone ecosystem, flood-plains and heath forest will be described in between the major ecosystems.
Figure 3 compares some ecosystems in Malaysia. The mangrove have fewer number of plant species as compared to the lowland forest. The sea which is another ecosystem found in Malaysia is different from the river.




As one goes southward from the north pole or northward from the south pole, and gets nearer to the equator, there is more sunlight. In the northern hemisphere, like Russia some forms of boreal forest were found. This is also true for mainland China and the North American continent and Canada. Southward towards the equator, temperate forests are seen, where trees shed their leaves in the months of autumn, to prevent loss of water and prevent accumulation of snow during winter time. Nearer to the tropical belt, plants tend to remain greener longer.
Along the tropical belt, between 23oN and 23oS of the equator, many more plants species are able to flourish as more sunlight is readily available, for longer period of time, almost all year round. There is no winter/spring/summer/autumn season, but dry and wet seasons are sometimes quite clearly defined. Different types of trees dominate these areas. In turn, this will determine the regime of animal diversity able to survive within the ecosystem. Polar bears are found only in the cold polar region, together with seals, walruses and penguins. In the temperate forest there is richer plant diversity and animal diversity is also higher. The richest biodiversity is found within the ecosystems along the tropical belt. The best ecosystem and perhaps the richest is the tropical forest ecosystem. Interestingly, within the tropical rainforest one could find other smaller ecosystems such as the fresh water ecosystem comprising of rivers, swamps and lakes. There are also patches of areas with specific plant group dominating. As an example is large stand of bamboos or rattan. In open areas or gaps, pioneering trees such as Macaranga seems to dominate. The presence of fast growing, sun loving and dense-leaved Macaranga is a blessing for it provides shade for the dipterocarp seedlings which do not favour sunlight. However, generally in pristine primary rainforests of Malaysia, one finds a closed canopy condition with large tall trees growing apart from one another, and few undergrowths, allowing for easy access. It is normally shaded and cool, humid and pleasant.
Although Malaysia is a comparatively small country in the world: 0.2% of global land mass, lying near the equator, it has several different types of ecosystems. To facilitate reading of this article, the classification of the various ecosystems will follow that of Whitmore 1998 [3] and also MOSTI 1997 [4].
The classification of natural ecosystem in Malaysia is determined by the presence of water body. Therefore, the first division is the aquatic and terrestrial ecosystems. Among that, in the aquatic ecosystems are the freshwater ecosystem and the marine ecosystem. For freshwater, there are the running riverine ecosystems (Fig. 4) and the standing freshwater lakes. In addition, there is also the mangrove ecosystem that line seashore and rivers (Fig. 5); and peat swamp ecosystem some of which is inland and with standing water body.


The largest ecosystem in the world is the marine ecosystem. This ecosystem covers about 70% of the surface of planet earth. There are several major oceans : the Atlantic Ocean, Pacific Ocean, Indian Ocean. Then we have major seas such as South China Sea and Mediterranean Sea. Water in the ecosystem is salty due to the high sodium chloride content. Peninsular Malaysia is separated from the two other Malaysian states on the island of Borneo by the South China Sea (Fig. 6).

The first discussed in this article will be the marine ecosystem followed by other ecosystems that are associated with water. Then the terrestrial ecosystem will be discussed beginning with a description of the tropical rainforest as a whole before beginning with the lowland tropical rainforest of Malaysia. Characteristics of each ecosystem will be described, illustrated basically by the various dominant vegetation groups. Peculiar animals found in the ecosystem will be elaborated. Then the importance or values of the ecosystem to the local, national and global communities will be highlighted. The final aspect to be discussed will be the threats for each of the ecosystem, which would naturally involve discussion of the conservation efforts.
Table 1 shows some facts and figures regarding aquatic ecosystems in Malaysia as compared to the world. Malaysia has 9,323km of coastline, whereas globally the total length of coastline is 1,634,701km.
Table 1: Fast facts of coastal and marine ecosystems in Malaysia [5].
| Category | Malaysia | World |
| Length of coastline | 9,323 km | 1,634,701 km |
| Percent of population within 100 km of the coast | 98% | 39% |
| Area of continental shelf | 335,914 km2 | 24,285,959 km2 |
| Territorial sea (up to 12 nautical miles) | 152,367 km2 | 18,816,919 km2 |
| Claimed Exclusive Economic Zone | 198,173 km2 | 102,108,403 km2 |
| Area of mangrove forest | 1,659 km2 | 169,452 km2 |
| Percent of Mangrove forest protected | 7% | 13% |
| Number of Mangrove species | 36 | 70 |
| Number of Seagrass species | 9 | 58 |
| Number of Scleractinia Coral Genera | 72 | Not known |
| International Legal Net Trade in Live Coral | -130 | Not known |
| Number of Marine or Littoral Protected Areas* | 111 | 3,636 |
| Wetlands of International Importance* | 384 km2 | 730,116 km2 |
| Average annual capture of marine fish | 1,257,573 metric tons | 84,411,066 metric tons |
| Average annual capture of mollusks and crustaceans | 187,864 metric tons | 12,055,801 metric tons |
| Aquaculture production of marine and diadromous fish | 12,813 metric tons | 2,623,888 metric tons |
| Aquaculture production of mollusks and crustaceans | 70,244 metric tons | 9,889,688 metric tons |
1. MARINE ECOSYSTEM
The Earth consists of 71% water which makes it blue when Earth is viewed from the space. There are three large oceans that almost engulf the whole planet: Pacific, Indian and Atlantic. These three far-flung oceanic waters differ greatly in their abilities to support life. Many of their differences are traceable to variations in the Earth’s climate at different latitudes and to the shapes and positions of the present-day continents. The marine life being supported by these oceans is a phenomenon that gives seas their regional characters against global forces that would mix them and make them uniform. In addition to these three large oceans, listed below in Table 2 are the world’s other significant bodies of water.
Table 2: Main oceans and waters in the world [6].
| Body of water | Depth (m) | Body of water | Depth (m) |
| Pacific Ocean | 4,282 | Sea of Okhotsk | 838 |
| Southern Ocean | 4,000 | Sea of Cortez | 813 |
| Indian Ocean | 3,963 | Red Sea | 491 |
| Atlantic Ocean | 3,926 | Hudson Bay | 128 |
| Mediterranean Sea | 1,429 | North Sea | 94 |
| Sea of Japan | 1,350 | Baltic Sea | 55 |
| Arctic Ocean | 1,205 | Persian Gulf | 25 |
The ocean is a significant factor in influencing the world’s global climate pattern. Phenomena like the El Niño Southern Oscillation (ENSO) is a product of changes originating in the Pacific Ocean significantly affecting the contours of continents and the lives of the people who inhabit coastal areas.
The productivity of the ocean cannot be underestimated. Marine organisms are important food source for the human population and provide resources for industry and medicine.
Marine ecosystem encompasses varied environments such as oceans, salt marsh, intertidal areas, estuaries, lagoons, mangroves, coral reefs, deep sea, and the sea floor. These ecosystems have high salt contents as compared to freshwater ecosystem (i.e. ponds, lakes, rivers) with lower salt content.
Similar to terrestrial (land) ecosystems, the marine environment consists of two general external factors: physical (abiotic factors) and biological (biotic factors). The physical environment consists of non-living components such as temperature, salinity, pH, and the amount of sunlight that penetrates the water, ocean current, wave action, and type and size of sediments. The biological factors on the other hand include living organisms and their interactions with each other. The ability of an organism to tolerate changes in the physical environment and to co-exist with other biological factors determines the organism’s distribution and diversity in the marine ecosystem.
Marine Microbes
Viruses and Bacteria
Like terrestrial environment, the marine ecosystem is home to thousands of species, ranging from bacteria and protists to plants and animals. Bacteria and protists provide indispensable life support for larger animals that most of us are familiar with. Table 3 summarizes the minute organisms found in marine ecosystems and the important roles they play.
Table 3: Summary of microscopic organisms found in marine ecosystem [7].
| Taxonomic group | Representative organism | Form and function | Ecological roles |
| Viruses | Bateriophages, cyanophages, phycoviruses, podoviruses, retroviruses, papillomaviruses, morbilliviruses | Icosahedral or helical as free virons, with protein capsid surrounding the genome; some with envelope from host cell membrane | Parasitic, major cause of mortality of among plankton, possible control of bloom-forming microbes, important in alteration of pelagic food webs |
| Bacteria | Blue-green bacteria, green and purple sulfur and non-sulfur bacteria, actinobacteria | Prokaryotic with cell wall containing aminated sugars; some flagellated; shapes mostly include rods and spheres, some mycelia | Chemoautotrophs and photoautotrophs, decomposers, nitrogen fixers, symbionts for light production or nutrition; form stromatolites with other microbes |
| Archaeons | Halobacteria | Prokaryotic with cell wall lacking aminated sugars | Chemoautotrophs and photoautotrophs, heterotrophs, methanogens; inhabitants of extreme environments |
| Fungi | Ascomycetes, including yeasts; lichens | Eukaryotic with cell wall of chitin; single-celled as yeasts or forming mycelium of hyphae | Heterotrophs, decomposers, symbiotic with green algae or blue-green bacteria in lichens |
| Stramenopiles | Diatoms, silicoflagellates, labyrinthulids, brown algae | Eukaryotic, heterokont flagella, one with hair-like filaments; diatoms with frustules of pectin and silica | Photoautotrophs, some forming harmful blooms, heteroautotrophic decomposers; producers of seagrass wasting disease; form deep-sea deposits |
| Haptophytes | Coccolitophoes | Eukaryotic; two-simple flagella with haptonema at base; cell covering of calcium carbonate plates | Photoautotrophs and phagocytic heterotrophs in plankton; for deep-sea deposits |
| Alveolates | Dinoflagellates; ciliates, including tintinnids | Eukaryotic; pellicle of membranous sacs; no cell wall but some with loosely fitting lorica; two heterokont flagella, or many cilia; cilia often fused as membranelles | Photoautotrophs, osmotrophs, phagotrophs; planktonic and benthic; some symbiotic as zooxanthellae or forming harmful algal blooms |
| Choanoflagellates | Choanoflagellates | Eukaryotic; one-flagellum with hairlike filaments, collar of microvilli; in gelatinous or branched colonies or as single cells with lorica | Planktonic and benthic filter feeders on bacteria |
| Foraminiferans | Forams | Eukaryotic; reticulopods; test of calcium carbonate or foreign particles | Planktonic suspension feeders and benthic grazers; phagocytic; form deep-sea deposits |
| Actinipods | Radiolarians, acantharians, heliozoans | Eukaryotic; perforated organic capsule, actinopods; internal skeleton of silica or strontium sulfate | Planktonic suspension feeders and benthic grazers; phagocytic; form deep-sea deposits |
Marine Plants – Seaweeds
Seaweed is a general or commonly used term applies to any apparently unwanted plants that are found in the sea that include the macroscopic algae and sea-grasses (angiosperms). In fact they are not unwanted or not useful but their uses are not yet known or realized. They are the only kind of plants found in marine environment. Technically, seaweeds belong to the group of macroscopic, multicellular algae such as the red algae, green algae and brown algae. Seaweeds are also called marine macroalgae. Seaweeds contribute substantially to primary production and are consumed by a wide variety of animals, particularly sea urchins, snails and fishes [7].
Seaweeds are benthic organisms, commonly found growing on a variety of environment like rocks, sand, mud, coral on the sea bottom, and so forth. Some seaweeds attach to very specific surfaces, whereas others are non-selective. In general, seaweeds inhabit about 2% of the seafloor [7].
Today many seaweeds are found very useful and important not only for the ecological integrity of the marine ecosystem but also as economic products. Seaweeds are known to have economic value and many species are consumed as food as well as medicinal and pharmaceutical applications.[8] In as early as 300BC, sea weeds have been known to treat glandular disorders, such as goiter.[7] Other medicinal application of sea weeds is for treatment of burns and rashes.
Marine Plants – Seagrasses
One of the most common marine plants is sea-grass. They are flowering plants or angiosperm, that grows in shallow sea bottom either on the muddy substratum or even sea rocks. In Peninsular Malaysia they are easily observed in the shallow sea of both sea-shores of Johor, around the islands off Mersing and also the island off Pontian.
Seagrasses are “hydrophytes”, which means they generally live beneath the water. In contrast to marsh grasses and mangroves, which require periodic exposure to ebb tides to grow and reproduce, seagrasses can grow, flower, fruit and germinate the seed while fully submerged. Among flowering plants, they are best adapted to marine life. There are about 66 species of seagrasses in the world,[7] although the majority of the species are found in the tropics (examples being Thalassia, Halodule, Syringodium) [6].
Seagrasses are food sources for herbivores, detritus feeders and bacteria. Moreover, seagrasses are important in depositing and stabilising coastal sediments, and seagrass beds provide habitats for many marine species. In addition to their ecological importance, seagrasses are source of food, medicine and other industrial applications. Some native cultures eat seagrass seeds and grind rhizomes into flour for their starch content. Sea-grasses are commonly used as herbal remedies for rheumatism and skin ailments. Also, sea-grasses are harvested as peat fuels – burning of peat allows extraction of minerals from sea-grasses [6][7].
Marine Plants – Coral reefs
Coral reefs are massive accumulations of reef limestone, a form of calcium carbonate built by coral polyps, encrusting red algae, calcareous green algae and many other organisms. While walking along the beach/sea shore you might be able to see coral such as this (Fig. 7).

There are three general forms of reefs in tropical oceans. These are (i) fringing reef – a coral platform built right up against the shore of a volcanic island; (ii) barrier reef – which encircles a volcanic island and is separated from the shore by a shallow lagoon; and (iii) atoll – a huge low-lying circles of coral enclosing a lagoon with no central island [9]. The famous coral reef island, Sipadan, on the east coast of Sabah is well known for its beauty and rich marine biodiversity (Fig. 8).

Attributed to Malaysia’s geographic location in the tropical region, it is marine ecosystem boasts as one of the most spectacular in the world, represented by the Andaman Sea (which borders the Indian ocean), the Malacca Strait and the South China Sea.
Unique marine animals of Malaysia
Large marine mammals found in the Malaysia water include the whale shark and dugong. Dugongs are large marine mammals that feed on seagrasses of the tropical ocean. During ancient time they are said to be mermaids, a sea creature with upper human body and a lower fish body. As for whale shark they predates on smaller marine life. In the sea of the south eastern part of Sabah, example being the Cowie Bay, dolphins are also fairly commonly sighted.
Issues and Concerns of the Marine Ecosystems in Malaysia
a. Harmful Algal Blooms (HAB)
Many kinds of marine algae undergo rapid population increases under conditions that are still poorly understood. Some macroscopic algae like Caulerpa taxifolia have the capacity to expand its coverage of the seafloor. Microscopic algae on the other hand, often bloom as “red tides” and can wreak havoc in coastal fisheries by killing commercially valuable species. In worse cases, toxic species are especially notorious for their impact on populations of commercial fishes and shellfishes, eventually affecting populations of humans, birds, and marine mammals that consume them. Red tides are caused by population explosion of certain species of dinoflagellates, diatoms and blue-green bacteria. In addition, toxin-bearing cells can become air-borne as a result of rough sea-surface conditions and enter the lungs of marine mammals and humans when they inhale the mist, causing them respiratory distress and even death. Toxic marine HAB are caused mostly by dinoflagellates. The toxins they produce are not part of their normal metabolism but are specially produced for protections. Such chemicals (i.e. secondary metabolites) are highly complex end products of elaborate metabolic pathways that function under altered growth conditions. The best known HAB toxins are the “saxitoxins”, a family of neurotoxins produced by dinoflagellates. The human syndrome caused by consumption of saxitoxins is called “paralytic shellfish poisoning”. Other human syndromes caused by other dinoflagellate toxins include diarrhetic shellfish poisoning, neurotoxic shellfish poisoning, and ciguatera fish poisoning, the latter caused by benthic dinoflagellates. Another syndrome – amnesic shellfish poisoning, is caused by toxin produced by blooms of diatoms [7].
b. Unsustainable harvesting
There has been concern on the ways fishermen harvest marine organisms. Although most abide to rules and regulation by the authority, there were cases of fishermen using unsustainable harvesting methods such as fish bombing and poisoning. Poisons such as cyanide and a local plant based chemicals the tuba (from plant of genus Derris) were used to collect high value fish species. Harsher penalty and more frequent monitoring of such activities are now being implemented to prevent unsustainability of marine resources.
c. Sea pollution
Malaysia provides for an active passageway for marine trading since ancient time. As such, its water is exposed to several polluting activities such as oil spills and dumping of toxic and solid waste. Sometimes, the solid waste garbage is visible along beaches. In addition, ocean water may be polluted by oil spill from ships. Another source of pollution is the toxic materials that originate from industries along river banks. Figure 9 shows solid waste pollution on a beach, comprising mainly of plastic bags and bottles.

d. Erosion of coastal line
In some areas such as the Tanjung Piai, the mangrove stand are facing growth problem. This is due to the forceful waves caused by the ships passing by the straits of Malacca. The mangrove stand tends to be fragmented as shown in Fig.10. Mangrove is important in coastal protection and in Malaysia after the Tsunami incident of 2004, efforts are being made to replant mangrove vegetation [9]. In the north-eastern part of Malaysia, in the state of Kelantan, erosion of the coastline are easily observed on two once famous beaches of Pantai Cahaya Bulan and Pantai Dasar. Thirty years ago, during the 1980s, the coast lines were some 50 metres from the present coast lines.

Reefs conservation Issues
Given that marine ecosystem is recognised as one of the most important ecosystem in the world, efforts to conserve it have been widely exerted. In Southeast Asia, only 10% of the total marine ecosystem is protected in one way or another; 88% of all coral reefs are under threat. Importance of reef includes being wave breaker and protecting shoreline from erosion. Reef fishes and other marine life found reef to be good homes and thus flourish well in the ecosystem. Coral reefs comprise of calcium carbonate, thus providing chemical balance in the sea-water. In terms of biodiversity, the coral reefs in Malaysian tropical water are one of the richest in the world. Those reefs in the vicinity of several islands, in particular Pulau Sipadan and Pulau Mabul, off the southern-east coast of Sabah; and those in Pulau Langkawi, off the north-western coast of Peninsular Malaysia and some islands on the east coast like Pulau Tioman, are amongst the spectacular ones.
2.BEACH FOREST
Marine ecosystem surrounds Peninsular Malaysia, most of Sabah and eastern part of Sarawak (Fig 11). Normally the coastal regions are sandy, especially the north and eastern coastline, with few vegetation due to the salty water of the sea. However, in certain areas especially in the western coastline, where rivers open up into the sea, large stand of special vegetation such as Nypa and mangrove that are able to survive brackish water flourish. These form the beach forest. Although, Nypa may be found among the beach forest vegetation, together with nibong palm (Oncosperma sp.) they form the normal elements of inland mangrove. Nipah palms (Nypa fruticans) normally line the banks of estuaries and rivers some kilometres inland; whereas nibong palms are found not only along the river banks but also on the banks of inland water bodies.
Along the north and eastern coastal lines of Malaysia, most are sandy with little vegetation except for the hardy coconut trees and Casuarina equisetifolia (ru). However, in areas where major rivers open up into the sea, some vegetation like Nypa and mangrove may be able to flourish. Generally at river mouths, the sedimentation created a muddy substratum.
The shoreline of Klias Peninsula, Sabah, is lined with mangrove and Nypa. The advantage of having such a shoreline is that the trees could act as wave breakers and lessen the amount of tidal forces that could erode the shoreline. A beach forest may comprise of large stands of beach forest vegetations and/or mangrove. Together with other beach vegetation like pokok kelapa or coconut trees (Cocos nucifera), Casuarina equisetifolia, with Dellinia suffruticosa and other palms, form a stable and strong barricade for huge waves that may otherwise erode the shoreline.( untuk kak ida)
Characteristic of vegetation groups.
The main vegetation present on this coastal strip will be those that could tolerate some level of salinity. Such trees belong to the mangrove group, Nypa and some others. If the dominant vegetation is of the mangrove group then the forest will be classified as mangrove forest. Beach forest will then be defined having other vegetation groups besides the mangrove. The typical beach forests consist of Casuarina equisetifolia on the sea front. As one goes a little inland one finds stands of jambu laut (the sea guava, Syzigium grande) and other species such as pandan laut (the sea pandanus, Pandanus tectorius), bintangor (Calophylum inophylum), penaga laut (the sea dragon, Colubrina asiatica), putat laut (Barringtonia asiatica) and several others. Some of the common herbs on the beach are Ipomoea pes-caprae, shrubby Scaevola taccada, the former originates from South America and now had become a weed on our beaches. Another species that could be found in a beach forest is Terminalia catappa (ketapang).
The trees are of various shapes and sizes. Some large trees such as Nypa, and coconut belong to the palm group. While Nypa and sago can thrive well in the brackish water of the river mouth, coconuts do need the sandy shore to anchor its fibrous root system. Casuarina with needle-like leaves line some shores. Almost all vegetation that is found along shores has leather like leaves, as this will prevent loss of water due to the wind and heat in these areas. The roots of the creeping herbs Ipomoea pes-capre bind the sand, hence inhibits beach erosion.
Importance of vegetation protection
Shore lined with trees is normally better protected than those without. During the events of tsunami on 26th December 2004 damage occurred heavily in Acheh and Thailand as well as Sri Lanka due to the absence of protecting vegetation such as the beach forest and mangrove. In Malaysia, the north western coast not barricaded by mangrove as in Kuala Kedah suffered much damage. Some parts that have dense mangrove and beach forest suffered lesser damage.
Malaysia has more than 4,809km of shoreline [10]. Much of it is sandy such as those of the north eastern coast of Peninsular Malaysia. However, there are stand of mangrove and beach forest along some tracts of the shoreline. Since the tsunami event that has caused much property damage and deaths in 2004, Malaysia is now implementing a programme called National Tree Planting Programme Along Coastal Areas. Dahlan et al., 2007 [9] describe the programme covering institutional framework, objectives, planning and implementation. They then reported the progress between 2005 to 2006. Several species of trees were chosen to be planted in the various state coastline. In Johor, Kedah, Kelantan, Perak and Selangor, Rhizophora apiculata, Rhizophora mucronata, Avicennia alba had grown well. In Terengganu, Pahang and Kelantan, Casuarina equisetifolia were generally successful despite some problems with grazing and monsoonal wind.
Thus, for sandy shores, beach forest may comprise of some species such as Cocos nucifera (coconut palm), Casuarina equisetifolia, Terminalia catappa, Syzygium grande, Calophylum inolum, Colubrina asiatica, Barringtonia asiatica and Dellinia suffruticosa among others. Some herbaceous plants are also common (Fig. 13); whereas muddy shores tend to be occupied by Nypa and mostly mangrove (Fig. 13).

2. FLOOD PLAIN and RIVERINE FOREST
Flood plains are flat land found along a riverine ecosystem and subjected to periodic flood event. Major rivers such as Sungai Kelantan in the north east of Peninsular Malaysia, has flat delta before the river opens up into the South China Sea. Periodically this flat delta is flooded especially during the monsoon periods, at the end of the year, and beginning of a new year. Globally, tropical floodplains are among the most productive landscapes, owing to their continual enrichment by the import and retention of nutrient-rich sediments from the headwaters and lateral sources. As such there is rich vegetation growing on this plain.
The best example of a flood plain is the Kinabatangan floodplain. Being the second longest river in Malaysia it has a span of 560km. Figure 14 shows the river running through central Sabah flowing eastward to Sandakan, towards the Sulu Sea. At the mouth of the river there is the huge mud flat where during rainy season will be flooded. The muddy soil of this plain contains many soil organisms including worms, insects and other invertebrates such as crabs. They are food for the waders and other birds. Reptiles also feed on them and thus one can occasionally see lizards and skinks running fast on the mud flats. Further up of this flood plain is the rich vegetation that grows on both sides of the river, forming the riverine forest. Plants growing in this ecosystem include those of the riverine forest and mangrove. The mangrove trees comprises of species of Rhizophora, Brugeira and Avicennia. There are Nypa trees in brackish water. According to Vaz 1997 [11], plant species of the riverine forest of Kinabatangan flood plain include Pterospernum javanicum, Colona serratifolia, Ficus spp., Lagerstoemia speciosa, Entada rheedii, Neonauclea spp. also some grasses and nomadic species Octomeles sumatrana (binuang).
It is known that the fauna of forest found along Sungai Kinabatangan is very rich. Vaz 1997 [11], reported on the fauna and flora of Kinabatangan that is summarized in Table 4. Worth noting is that all the 10 species of primate in Sabah occur in this area as well as all the 8 species of hornbills found in Borneo. In addition, the bats and birds life are also rich. Several species of small and medium sized mammals not easily found in other forested areas are also found in Kinabatangan. For this reason, the state government had cooperated with World Wildlife Fund for Nature (WWF) in designating parts of the riverine forest and floodplain of Kinabatangan as conservation areas. These conservation areas are designated as lots and there are 12 of them.
Table 4: A summary of fauna found at Sungai Kinabatangan, Sabah (from Vaz 1997) [11].
Mammals
| Primate | Large, medium and small size mammals | Bats | Birds | Hornbills |
| Nasalis larvatus (proboscis monkey) | Elephas maximus (Asian elephant) | (about 27 species) | (about 200 species) | Berenicornis comatus (White crested hornbill) |
| Presbytis cristata (silvered langur) | Dicerorrhinus sumatrensis (Sumatran rhino) | Rhyticeros undulates (Wreathed hornbill) | ||
| Presbytis rubicunda (maroon langur) | Cervus unicolor (Sambar deer) | Rhyticeros corrogatus (Wrinkled hornbill) | ||
| Presbytis hosei (Hose’s langur) | Tragulus napu (Greater mousedeer) | Anorrhinus galeritus (Bushy-crested hornbill) | ||
| Pongo pygmaeus (Orang utan) | Muntiacus antherodes (Barking deer) | Anthracoceros malayanus (Black hornbill) | ||
| Macaca fascicularis (Long tailed macaque) | Tragulus javanicus (Lesser mousedeer) | Rhinoplax vigil (Helmeted hornbill) | ||
| Macaca nemestrina (Pig-tailed macaque) | Bos javanicus (Tembadau) | Buceros rhinoceros (Rhinoceros hornbill) | ||
| Hylobates muelleri (Bornean gibbon) | Sus barbatus (Bearded pigs) | Anthrococeros coronatus (Pied hornbill) | ||
| Tarsius bancanus (Western tarsier) | Helarctos malayanus (Sun bear) | |||
| Nycticebus coucang (Slow loris) | Neofelis nebulosa (Clouded leopard) | |||
| Felis bengalensis (Leopard cat) | ||||
| Felis marmorta (Marbled cat) | ||||
| Felis planiceps (Flat headed cat) | ||||
| Lutra sumatrana (Hairy nosed otter) | ||||
| Aonyx cineria (Oriental small-clawed otter) | ||||
| Lutra perspicillata (Smooth otter) | ||||
| Mustella nudipes (Malay weasel) | ||||
| Martes fluvigula (Yellow-throated marten) | ||||
| Mydaus javanensis (Malay badger) | ||||
| Viverra tangalunga (Malay civet) | ||||
| Paradoxurus hermapohroditus (Common palm civet) | ||||
| Arctogalidia trivirgata (Small toothed palm civet) | ||||
| Hemigalus derbyanus (Banded palm civet) | ||||
| Echinosorex gymnurus (Moonrat) |
Clearly seen, from Table 4 Sungai Kinabatangan, a mudflat and flood plain ecosystem in Malaysia is rich in biodiversity. Efforts had been initiated to conserve the riverine ecosystem through the establishment of several plots of land along the river bank. Unfortunately, some of these plots are not continuous (Fig. 15) and thus create problems for conservation of migratory Bornean elephant. Lee, 2006 [12] reported on the human-elephant conflict arising from this situation. Nurzhafarina et al., 2008 [13] had studied the morphometrics of elephant in Kinabatangan, to establish the origin of the Bornean Asian elephant. Meanwhile, Rashid 2006 [14] studied the human-Orang utan conflict.
There are many different types of river ecosystems. Freshwater rivers range from small, clear, pristine streams that flow through elevated rocky terrain of primary forest to shallow fast flowing streams, with waterfalls, small pools, riffles and rapids. These rivers gradually grow into larger, slower, streams that merge into larger rivers and eventually into the sea. In some areas, these streams or rivers may accumulate into lakes at lower elevations. In major urban areas main rivers are often large, murky, sluggish flowing and highly polluted. Water chemistry plays an important role in regulating aquatic primary production in tropical floodplains.
The water of a river can be soft or hard (acidic or alkaline). Vegetation above river can affect organic load and temperature (shaded streams are cooler than rivers running through flat open lands). River bottom substrates can affect species composition. In rivers with muddy, sandy, silty substrates would have more benthic feeding organisms than those with rocky or clayey substrates.
Vegetation could be rich on muddy soils of the mudflats, such as Kinabatangan. According to Gisil et al., 2003 [15], Azmi 1998 [16] reported on the vegetation study by WWF Malaysia that listed 1,056 species from 129 families and 512 genera. From their study in 2002 at lower Kinabatangan, they added on another 24 new records of vascular plants. Figure 16 shows the river Kinabatangan from the village of Kg. Sukau.

Issues of rivers
Rivers were traditionally used for navigation and water transport. They are also sources for fishes and other aquatic food. However in many instances river are used as dumping grounds for domestic, agricultural and even industrial wastes so much so many of them become polluted. As in many areas in the world, Malaysia has its share of river water pollution problems. Reports show that many major rivers are of poor quality. The Malaysian government is seriously trying to solve this problem. Besides rehabilitating the rivers, Malaysian water agency/authority is working closely with non-governmental organizations to educate people on their responsibility in ensuring that rivers in Malaysia are taken care of. One such programme implemented in 1993, is the Sayangi Sungai Kita (Love Our River), as seen from Fig. 17.
A study of Kinabatangan also indicated that this river is polluted [17]. Pollution comes from logging activities upstream and agrochemicals used in the plantations lining the river banks. Naturally, this situation will affect biodiversity of the river ecosystem.

3. MANGROVE
The origin of the English word “mangrove” is uncertain and there are various theories relating to its etymological evolution. It may in part be derived from the old Malay manggi-manggi, used to denote a specific family of mangrove trees and still used today in eastern Indonesia to describe the genus Avicennia. Members of this genus were named el gurm (or el q’urum) by the Arabs and it is suggested that the word “mangrove” may have derived from the term mang-gurm, a combined form of the old Malay and Arabic names. Other theories relate the term to a composite word of native American and Portuguese or Spanish origin, or even a simple derivation from the English word “grove” used, during colonial times, to denote a dense stand of tropical trees.
The term “mangrove” can either be used to denote an individual plant in a mangrove community, or the entire community itself consisting of biotic components, their inter- and intra-specific interactions, and their overall environmental functioning. Thus, in its broadest sense the term “mangrove” can be used to describe any vascular plant or plant community that occurs in areas subject to periodic fresh- and salt-water inundation. However, in other literatures, the term “mangal” relates to the functional community and mangrove is served for individual plants.
Mangrove forests are restricted in their geographical range to the tropical belt between the Tropic of Cancer to the North and the Tropic of Capricorn to the South. Their distribution is limited to areas where the mean annual temperature of coastal surface is above 16°C. To date, there is no exact total area of mangrove forests worldwide, but the figure is between 14.2 to 25 million hectares.
| The term mangue, manguezal or manglares were commonly used by Portuguese and Spanish seafarers to describe all the varieties of coastal and swamp vegetation they came across in the lands they conquered. Since these seafaring nations were constantly in contact with Arabic and Malayan cultures long before they sailed to the West Indies and Brazil, they most likely adopted the word mangue from mang-gurm. |
Figure 18 shows a typical mangrove vegetation. These vegetations have adapted themselves to the brackish or salty condition. In addition, due to being submerged periodically, they have also evolved different forms of root systems to survive despite being submerged periodically.

Special Characteristics of Mangrove Plants – Root Systems
Mangrove plants are different from other plants. They have various morphological features and adaptive mechanisms in common which allow them to grow in periodically saline environments. Halophytes are salt-tolerant plants and mangroves are salt-tolerant plants; however please note that this does not mean that mangroves need salty environment to survive.
The most conspicuous feature of mangroves is their root formation. It reflects enormous adaptability of this vegetation to survive in relatively adverse conditions. Various root systems have been developed by different species to provide the trees with sufficient stability in muddy bottoms and simultaneously function as supplementary respiratory organs.
The following are the types of root system of mangroves:
- Stilt roots – arch-shaped, strong roots, often like flying buttresses branching from the lower part of the main trunk. Being secondarily branched they give the tree optional stability. Example: Rhizophora
- Aerial (Prop) roots – unbranched, few centimetre in diametre adventitious smooth roots growing downwards from large branches, sometimes from the canopy to the bottom. In the latter case they may act as additional support against tilting. Example: Rhizophora, Avicennia, Acanthus
- Respiratory roots (Pneumatophores) – erect pencil or arm-like roots being upward of a radial subterranean root system. Pneumatophores are usually unbranched; they exist principally for respiratory purposes. Example: Avicennia, Sonneratia, Languncularia
- Cable (Plank) roots – fingerlike, smooth, sometimes plank-shaped roots which radiate in a sinuous course partly above, partly ground. Example: Xylocarpus, Heritiera
- Knee roots – modified sections of cable roots, forming pronounced upward loops before horizontal growth continues; these knee-like outgrowths also have respiratory functions. Example: Bruguiera, Ceriops
- Buttress roots – similar to stilt roots, but expanding at the parts connecting with trunk base into flattened and plant-like support structures. Example: Heritiera
- Flute buttresses – organ/pipe-shaped buttresses at the trunk bases formed by enclosed aerial roots; fused together into kind of conical trunk. Example: Perriciera
- Creeping roots – Dichotomously growing protrusions (primary roots) at the lower parts of the main stem, growing horizontally just below surface. Example: Nypa
Another unique feature of mangrove plants is their special reproduction strategy. In order to survive successfully, mangroves have to master their amphibious environment. Firstly, in a habitat with slow diversity of potential pollinators they have to overcome problems association with pollination. To cope up with the relative scarcity of pollinating insects, mangroves commonly synchronise their flowering season with annual changes in climate, each species in a particular region having its own flowering period. To maximise the number of their potential pollinators some mangrove flowers develop fragrance only during night. Others seem to attract, either by colour or scent, insects or sunbirds which are active during daytime; still others produce flowers mainly during the dry season thus avoiding competition with terrestrial flowering plants and performing an important service for nectar-eating sunbirds which use terrestrial herbs as their main food during the wet season. Some mangrove of the genera Sonneratia and Bruguiera have adapted their flower structure for pollination by particular birds and bats. They form heavy flower cups with stout sepals allowing the visitors to cling with their claws. In addition, mangroves which depend on animal vectors for pollination, have developed pollen with small hair or a sticky coat.
The second reproductive adaptation of mangrove is that plantlets have to survive in unstable soils or for long periods in salt water. And once seedlings become established, it must survive attack of various herbivores and encrusting aquatic organisms such as algae and barnacles and endure the problems of strong currents and intensive sedimentation. To adapt to this challenging environment, mangroves particularly of the family Rhizophoraceae have successfully developed “vivipary”. In viviparous species the seed germinates whilst it is still attached to the mother tree so that each dispersed seedling is already well developed and has a better chance of survival. Vivipary has obvious advantages for species which live in such adverse conditions as mangroves: when seedlings has drifted or fallen into a suitable area it can root quickly and thus survive in the battle against soft sediments and fluctuating tides.
Viviparous Rhizophora seedlings have a characteristic torpedo shape and is some species these propagules may be as long as 80 cm (Fig. 19). When a propagule is detached from the mother tree during low tide is either falls vertically and simply sticks into the exposed mud where it begins to grow, or falls from the mother tree into water and is then carried away with the currents. All is not lost however, as the propagules can withstand immersion for long periods in saltwater and may drift for long distances before setting on some distant shore. Initially each propagule floats horizontally but after several weeks the pointed end gradually absorbs water and the seedling becomes vertical. When this happens the seedling produces its first leaf shoots from the top end and its first roots from the bottom so that, as soon as it settles, it sprouts small anchoring roots and new leaves.


Fig 19: This kind of torpedo shaped matured seed will facilitate anchoring of the germinating into the muddy and soft soil of the mangrove ecosystem.
However, there are mangrove species that do not have this characteristic of vivipary. One example is the Lumnitzera sp. (Fig. 20).

Fig. 20: Lumnitzera sp. is a group of mangrove plants that do not exhibit vivipary.
Common animals in mangroves
Animals associated with mangrove ecosystem can be more or less divided into aquatic and the arboreal. Most of the aquatic organisms are of the marine origin, like mollusks, crustaceans and fish. Meanwhile the arboreal groups they comprise of many more animal groups from invertebrates such as insects to mammals, many of which never actually come in contact with tidal waters. Others such as birds often rely on the mudflats and creeks rather than the trees. Mammals are largely arboreal although, some do make their home in the canals and lagoons in these ecosystems. Listed below are the common fauna in mangroves.
- Zooplankton – most zooplankton drifting through mangrove waters originate from coastal marine waters. Copepod crustaceans are dominant both in number of species and in biomass. Zooplankton is the main food source for filter feeding organisms like oysters, mussels, sponges, crabs, shrimps and fishes.
- Worms – the upper 10 to 20 cm of mangrove sediments are ideal habitat for various marine worms, all of which are well adapted to conditions of low oxygen, high soil acidity and fluctuating salt concentrations. The most common mangrove-dwelling worms are nematodes, polychaetes and annelids. Their significance to the ecosystem is in their role as a source of animal protein for higher organisms and their contribution as decomposers of organic detritus.
- Univalve (gastropods) and bivalve mollusks – gastropods and bivalves associated with mangrove wetlands are also found on or in other tropical near shore marine environments (soft bottoms, rocky intertidal, reef platforms, coral reefs, sea-grass flats, etc.). The most common gastropods and bivalves are ship-worms (Teredinidae), mud snails, pulmonate snails, oysters, mussels, cockles, arkshells, tellinids, and clams. Fig. 21 shows some shells of mollusk in a mangrove ecosystem.

- Crustaceans – crustaceans are the most versatile creatures of mangrove wetlands, occupying virtually all microhabitats in this ecosystem. Some are, except during their planktonic phase, completely sessile and fixed to a calcareous plate attached to a mangrove root. Others are fast-swimming predators dashing through creeks and lagoons. The most popular crustacean is Macrobrachium – the giant freshwater prawn. The life cycle of this freshwater prawn is similar to that of their marine relatives, the major difference being that adult freshwater prawns migrate out of mangrove water in an upstream direction in order to reach their individual mating grounds. Crabs are also a type of crustacean in mangroves and the most common is the fiddler crab (Uca sp.) as in Fig. 22.

Fig. 22: This is a fiddler crab of the mangrove ecosystem; note the huge size of its pincers.
- Fish – since mangroves experience high and low tides, the most prominent species of fish are the mud skippers (Periophthalmus spp.) although other species are also found like butterfly fish, damselfish, anemone fish, blennies, gobies, goatfish, sea horses, and rabbit fish. Fig. 23 shows al photograph of a mudskipper, unique to the mangrove ecosystem.

Fig. 23: Mudskippers are fishes that are able to be outside water for a substantially long period of time.
- Insects – the relative scarcity of insect species in mangrove is prominent and some of the few striking ones are ants, termites and pollinating hymenopterans (wasps and bees). Termites play significant role in mangrove forests through their removal and decomposition of rotten wood and decaying branches. One of the most important insects in mangroves are fireflies – providing spectacular nocturnal phenomenon unique to mangrove forests. Only males of certain species of fireflies send out synchronised light pulses at frequencies that vary between species, from the slowest at about 0.7 flash cycles per second, to the fastest at around 13 per second.
- Reptiles – In general, most reptiles find it difficult to live in saltwater because it is not easy for them to excrete excess salts from their bodies, however there are also those reptiles that are able to adapt like saltwater crocodile (Crocodylus porosus – Fig. 24), snakes like viper and dog-faced cat snakes, marine turtles like the leatherback (Dermochelys coriacea), and monitor lizard (Varanus salvator).

Fig 24: This species is the Crocodylus porosus and commonly called the estuarine crocodile.
- Birds – water-birds abound in all mangrove areas. This highly productive ecosystem ensures a plentiful food supply for herons, egrets, spoonbills, shorebirds, gulls and terns. In addition, undisturbed mangrove swamps provide ideal nest sites for the resident herons, ibises, egrets, pelicans and cormorants, which often form spectacular colonies on treetops. Mangroves and their associated intertidal flats are also home to many species of migratory shorebird, an example the white egret (Fig. 25). Many of the species which nest in the Northern Hemisphere during the summer months and fly south for the winter rely on these areas for refueling during the long migration and, during these periods, tens of thousands of shorebirds can be seen in mangrove wetlands, each species occupying the habitat which best meets its needs.

Fig. 25: Migratory birds include the white egret, Egretta sacra.
- Mammals – mangrove wetlands are home to some specialised mammals that are either skilful climbers, such as leaf monkeys, or versatile swimmers such as otters. Other common mammals common in mangrove areas are rodents or water rats (Xeromys). The extensive mangrove swamps of Borneo are home to one of the most bizarre looking of all mammals, the proboscis monkey (Nasalis larvatus). Figure 26 shows the male proboscis monkey has long and pendulous nose. There is normally one male in a harem of females. This species, Nasalis larvatus is endemic to Borneo Island.

Fig. 26: The male proboscis monkey, Nasalis larvatus.
The many uses of mangroves
Mangrove wetlands offer an enormous variety of natural products, used daily, form part of the traditional lifestyle of the people living in these regions. When we take into account the traditional knowledge of mangrove dwellers from all over the world, it is amazing to discover how many drugs and medicines – prophylactics, intoxicants, beautifiers or aphrodisiacs – are derived from mangrove plants. For example, Nypa is most versatile and useful plant in the mangrove forest. Practically, all parts of this plant can be used to provide a supply of basic goods, ranging from construction and fuel materials to food, drugs, and various good used in rural industries. The young shoots, decayed woods and leaves of this plant is used in Southeast Asia to cure herpes, toothache and migraine, the outer layers of the leaf stalk yield pulp suitable for good quality boards, while the outer layers of young shoots are converted into cigarette wrappers (e.g. in Malaysia). When burnt, these shoots produce useful mineral salts. In Malaysia, Indonesia and the Philippines, there are still existing distilleries producing local wine called “toddy”, fermented from Nypa sap.
Most common uses of mangrove plants:
- Food items – in some regions of Southeast Asia and Colombia, the seeds of Avicennia are eaten. In Malaysia and Indonesia people prepare the leaves and fruits of Sonneratia as vegetables, while in Thailand they relish the root tips of Bruguiera. Others consider a salad made of sprouts of Oncosperma tree a delicacy. Indians and Sri Lankans use the mangrove fern Acrostichum for similar dishes. In Indonesia, the leaves of Rhizophora are sometimes cooked with fish to acidify the meat. Xylocarpus seeds are said to give a pleasant, bitter taste in local brandy. Nypa fruits, tasting somewhat like unripe coconut meat, are a welcome dessert in the daily meals of mangrove dwellers. In Fig. 27 is shown a fern found in mangrove that can be eaten.

Fig. 27: Some vegetation in mangrove are edible such as this fern known as piai (Acrostichum aureum).
- Medicinal plants – Bruguiera species, distributed all over the Indo-Pacific, provide a number of useful drugs. The scraped skin on the fruit is sometimes applied to stop bleeding and the fruit extract of some species is considered an ophthalmic remedy. The bark is sometimes used as a cooking spice. Another remarkable medical use is reported from India where leaves of Bruguiera are prescribed to control blood pressure. The traditional medicine of Fiji islanders includes many curative applications of mangroves. One notion is that the smoke of Excoecaria agallocha bark accelerates the healing of leprosy. Another mangrove (Cerbera odollam) is believed to alleviate toothache when mashed leaves are stuffed into dental cavities. In various countries in Southeast Asia, the kernels and bark of the same mangrove provide oil extracts which are applied medicinally. The sap of Cerbera serves as purgative and haemostat, while rubbing the limbs with the fruit extract is said to bring relief from rheumatic pains. Extracts from Rhizophora propagules or from leaves of Acrostichum ferns can also be applied to stop bleeding. In cases of sore throat and stomatitis people of the Pacific coast of Colombia prepare a gargle from bark infusions of the red mangrove (Rhizophora mange). In Indonesia and Thailand people believe that extracts of Heritiera fomes seeds or Ceriops bark ameliorate attacks of malaria fever. Rural households in southern Thailand often fuel their small lanterns with oil extracted from the cannonball-shaped Xylocarpus fruit. In Indonesia, the oil serves as a pomade and mixed with rice flour as a face mask. This yellow mixture is also used as a cure for acne. Some Asians use it as mosquito repellent. In the Philippines, fruit extracts are also employed to treat insect bites and dysenteric fever. Figure 28 shows leaves of Rhizophora sp. [18].

- Industrial products – mangrove forests also offer a number of essential raw materials for local industries. For example, mangrove tannins and resins provide the basis for the production of ink, battery water, detergents, rubber solutions and glues. Rhizophora woods is used in Malaysia as foundation moulds for concrete in the construction of tall buildings. The high tannin content prevents rotting. Other products made of mangrove pulp are cardboard and newspapers. In Bangladesh, about half of the national paper production originates from Heritiera fomes and Excoecaria agallocha. Various species of the Avicennia and the universal Nypa palm are suitable sources of paper pulp, while the wood of Rhizophora is used mainly in the manufacture of chipboard and synthetic fibres (e.g. rayon). During the last decade, this particular industry, mostly undertaken by Japanese companies, has sustained various coastal regions in the Philippines, Papua New Guinea and Indonesia but only at great ecological cost. Another use of the Rhizophora wood is charcoal making.
- Tannin – in ancient times, Chinese and Arabs mastered the technique of preserving leather skins with extracts from mangrove bark. With the boom in the leather industry in the middle of the last century, and during the period of European colonization, the import of mangrove tannin became a thriving trade. Between year 1890 to 1900, about 1,350 tons of mangrove barks were shipped out each year, mainly from the German colonies in East Africa. By 1928, the annual German tannin production has used about 10,000 tons of mangrove bark, all obtained from a single region in Madagascar which was totally depleted. After World War II, the demand for natural tannins was reduced drastically due to the development of cheap synthetic tannic acids. At present, the tanning of leather with fresh bark extracts (mostly Rhizophora and Ceriops) is practiced in only a few countries in Latin America. In Polynesia and East Africa, local people still dye their tapas, the typical cloth made of bast, with mangrove tannins. Artisanal fishermen in all tropical seas still preserve their cotton sails and natural fibre ropes with bark extracts from mangrove.
- Forestry/timber – many mangrove timbers possess qualities which make them suitable for specific wood uses. Typical mangrove timber is heavy, durable and finely grained; annual rings are absent or rarely visible. Some timbers are excellent for carving, others produce pleasant scent, while some species are used as fuel-wood.
Characteristics of mangrove ecosystem
Normally mangrove ecosystems are found near river mouth opening into the sea (estuaries) and along sheltered tropical coast. The water in which the mangrove trees grow is salty; or alternating salty and brackish depending on the tides. Trees that grow in this ecosystem, are normally not very tall, reaching to about 10m. Those that grow in the salty water are normally the Rhizophora group, Avicennia and Lumnitzera.
4. INLAND WATER
Inland water of Malaysia is mainly of freshwater ecosystem. Table 5 shows the inland aquatic ecosystems in Malaysia.
Table 5: Malaysian inland water sources as mentioned by Dahlan, et al., 2007 [12], sourced from : Yusoff and Gopinath, 1995 [19].
| Habitat | Area (km2) |
| Peninsular Malaysia | |
| Rivers including flood plains | 9,111 |
| Peat swamps | 4,850 |
| Reservoirs | 1,600 |
| Mining pools | 164 |
| East Malaysia | |
| Rivers including flood plains | 8,487 |
| Peat swamps | 15,150 |
| Reservoirs | 22 |
Some large lakes in Malaysia include Tasek Bera and Tasek Chini. Tasek Bera, in Pahang (Peninsular Malaysia) is the first RAMSAR site in Malaysia. There are now six RAMSAR sites in Malaysia.
5. PEAT SWAMP
Tropical peat lands are found throughout the tropics, however the largest acreage is in South East Asia, having about 20million ha mainly in the coastal lowlands of Borneo, Sarawak, Indonesia and Peninsular Malaysia [20]. In Malaysia there is about 1.54mill ha (<20% Peninsular Malaysia, over 70% in Sarawak and the rest in Sabah). Due to demand of land for the various land uses, acreage of peat swamp in the world is decreasing, so it is in Malaysia. Taking Peninsular Malaysia as an example, Ongkili 2005 [21] stated that in 1981 the peat swamp forest was estimated to be about 670,000ha but by 2005, it has declined to 200,000. However, he noted that the remaining peat swamps is largely classified as Permanent Forest Reserves and will not continue to decline.
FORMATION OF PEAT
The formation of peat is interesting. Fallen trees were not fully decomposed or very slowly decomposed, perhaps due to poor decomposer fauna, such as insects, fungi and bacteria. This may happen when trees fell into salty water collection/pool. As more fallen trees accumulate a layer of partially decomposing vegetation appear and this is called peat. This peat layer is made up of trees at various stages of decomposition. In Fig. 30, is a picture of the peat swamp forest of Klias in Sabah.

USES OF PEAT SWAMP FOREST
Uses of peat swamp are many, including in flood mitigation and harbouring unique biodiversity. Since the water of peat swamp is acidic, plants that survive and thrive in this ecosystem are specially adapted to living in acidic condition. Like mangroves, peat swamps provide excellent breeding habitat for special organisms that has adapted to living in acidic water.
It is sad that currently peat swamp forests had been drained to make ways for vast areas of oil palm estates throughout Malaysia, especially in Peninsular Malaysia. In the state of Johor peat swamps were drained and cleared for pineapple plantations. Elsewhere in Pahang peat swamps had been converted to paddy fields and in the future peat swamp forests in Sarawak will become another “rice bowl” for Malaysia.
THREATS FOR PEAT SWAMP
Threats to peat swamp are many. The main being converting them to agricultural land and this is achieved through extensive draining process to dry the waterlogged ecosystem. When peat land is dried it provides a readily flammable condition that could be heightened with long drought. Upon ignition, by human activities such as burning of trash or burning cigarette butt or naturally through lightning, there will be fire that could spread easily because of the availability of dried decomposing organic materials underground. This kind of fire is not easy to manage as it burns underground.
CONSERVATION OF PEAT SWAMP IN MALAYSIA
Peat swamps in Sarawak are found along the coastal plain and is rain-fed. They have 65% organic content and are usually at least 50cm thick but can be as much as 20m deep. All incoming water is from rainfall, low in mineral nutrients, and drainage water is acidic with a pH of 4 or lower. Usually of tea colour, it is described as blackwater. Towards the centre of swamp the stream becomes more acidic, resulting in lower species abundance and diversity. Some species have adapted to this harsh environment; and often are restricted to peat swamp habitats. For instance the rare Arowana (Scleropages formosus) is found in the deep pool of peat swamp/rivers. Many fishes have evolved bright colouring to enable them to stand out in these dark waters and hence have become a favourite of many ornamental fish collectors [22].
PEAT SWAMP ECOSYSTEM – PENINSULAR MALAYSIA
In Peninsular Malaysia, the grant from United Nations Development Programme (UNDP) to conserve peat swamp was designated to South East Pahang Peat Swamp Forest Complex. It is believed to be the largest and least disturbed peat swamp forest in mainland Asia. It comprises of 200,000ha and located in four Permanent Forest Reserves: Pekan, Nenasi, Kedondong and Resak. It contains the full spectrum of habitats from shallow, coastal water to the rich mosaic of wetland and dryland habitats inlands. At least 221 of flora species have been recorded from peat swamp ecosystem here [27]. Significant flora species are: Ramin (Gonystylus bancanus), Durio catrinatus, Tetramerista glabra, Alstonia angustiloba, Nageia motleyi. Of the 17 taxa of tree endemics to Peninsular Malaysia (from Peat Swamp Forest ecosystem), 8 have been found in South East Pahang Peat Swamp Forest Complex.
As for fauna, there are least 62 species of mammals, 233 bird species, 8 reptiles and at least 56 species of fishes [20].
PEAT SWAMP ECOSYSTEM – SABAH
In Sabah the peat land is mainly in Klias Peninsular, Sugut and Kinabatangan floodplain. Two remaining protected peat swamp forests left, the Klias Forest Reserve (Klias FR) which is about 3,6000ha and the Binsulok Forest Reserve (Binsulok FR) about 12,106ha. Both are located in the Klias peninsular. In 2002 Sabah became one of the three recipients for a fund to conserve peat swamp forest in Malaysia. Management strategies were planned for and to obtain data that is needed for conservation, several studies were carried out. Universiti Malaysia Sabah carried out at least six studies under this project. These included a study to inventory the bird fauna of Klias, to document the insect diversity, study on the ecology of proboscis monkey, to set up GIS database, to look into the potential of eco-tourism, and to inventory useful plants [23].
As reported by Rashid et al., 2005 [24], in 2002 Sabah received a grant from UNDP/GEF Peat Swamp Forest project to primarily develop and implement plans, which encourage processes to ensure the conservation and sustainable use of globally significant genetic, species and ecosystem diversity within tropical peat swamps forest (PSF) and associated wetlands in three sites in Malaysia. Some of the outputs expected were information management system on biological diversity, establishment of biological diversity monitoring programme and development and demonstration of site specific plans for the respective states (Pahang, Sarawak and Sabah). Besides Sabah the other two sites are: Loagan Bunut in Sarawak and south eastern Pahang (FRIM-UNDP/GEF Peat Swamp Forest Project 2004). In Sabah, it was for a duration of five years from 2002 to 2007. To achieve its aim, several research projects were carried out to gain more information and understanding of the ecosystem.
Some studies have been done at these two forest reserves. Sato & Anton 2000 [25] found poor algal flora density, abundance and diversity from streams/rivers of both Klias and Binsuluk attributed to the very low pH of <4.0. Berhaman & Sugau (2000) [26] reported 95 species of 41 families of plants for Klias/Binsuluk FR., raising the previous record by Ong, et al., (1998) [27] to 134 species in 59 families. Dominants trees were Dryabalanops rappa (Seraya paya), Stemunurus scropioides, Palaquium rostratum, Gonystylus bancanus and Combretocarpus rotundus. In addition, Nepenthes spp. and the sealing wax palm Cyrtostachys renda (Fig. 31) were mentioned as having ornamental potential [26].
Takahashi & Berhaman (2000) [28] collected four species of Nepenthes from Klias (N. ampullaria, N. bicalcarata, N. gracilis and N. rafflesiana) and two (N. gracilis & N. rafflesiana) from Siunggau FR. Nishida & Maryati 2000 [29] conducted a preliminary study of the cuticular features in 11 angiosperm species showing that significant variations were observed in the ornamentation of epidermal cells, the structure of stomata and the presence of several types of trichomes. A comparison of features between certain species also revealed that cuticles can vary greatly within a family.

Mohd. Fairus et al., 2000 [30] found 66 species (42 genera) of butterflies (Lepidoptera: Rhopalocera). They represented all the five families found in Borneo. They noted the poorer butterfly fauna of the peat swamp and mangrove areas, corroborating the observation made by Corbet & Pendlebury, 1992 [31], Mohd. Nazri et al., 2000 [32] revealed that Cyprinidae was common for both Sg. Bukau of Klias and Sg. Mantabawan of Binsulok. Altogether, 23 species were sampled. Collection made at Klias yielded 22 species while there were only four from Binsulok. This was accounted for by the high acidity of Sg. Mantabawan, whereas from Klias, collections were made from both salt and fresh water habitats.
Maryati et al.,2000a [33] recorded 38 species of birds, eight netted and the rest visually observed. Most were common lowland birds of primary and secondary forests. Setornis corniger however, was found to be restricted to the primary forest. Yasuma et al., 2000 [34], collected eight species of small mammals, denoting a poor fauna diversity. This was attributed to the fact that most of the forest was submerged throughout the year. A total of 15 species of small mammals were trapped, with different species being ecologically separated in the three study sites; primary peat swamp forest, forest boundary and plantation areas.
The physical environment of the area was reported by Dalimin & Zulistiana (2000) [35] to have poor drainage and high acidity (pH 4.5) typical of a peat swamp environment. Quality of three elements were measured: water, soil and climate. An earlier study, had previously reported that soil type of Klias and Binsuluk was non-economic.[35] Then, Greer et al., 2005a [36] and Greer et al., 2005b [37] described the hydrological assessment of Klias Peninsular based on a two years monitoring data. It was found that development activities of the peat land surrounding Klias FR and in particular the drainage associated with conservation to agricultural land has been identified as a threat to the hydrological regimes of the forest reserve [36]. In the same section Deratil et al., 2005 [38], detailed out the organic component of the peat soil in Klias. They warned that when the peat is drained for agricultural crop cultivation, the top layer of organic matter quickly oxidizes, disintegrates and eventually disappears causing a subsidence. In addition, they noted that the inherent properties and characteristics of the peat soils of Binsulok and Klias severely limit agricultural crop cultivation.
6. DRYLAND FOREST
Introduction to a tropical rainforest
Tropical ecosystems, regions between the tropics of Cancer and Capricorn occur in three main blocks. Tropical America is the largest and has about half the total with a potential cover of roughly 3.0 million square kilometres, mostly in the Amazon and Orinoco basins. Southeast Asia comes second with approximately 2.0million square kilometres, in the Malay Archipelago extending into continental Southeast Asia as far as Sri Lanka and India to the west and into Queensland (Australia), Malenesia and Polynesia to the east. The third smallest rainforest is that of Africa, approximately 1.0million square kilometres, mainly in the Zaire basin of Central Africa and extending westward along the shores of Guinea.
Tropical forests account for approximately 60% of global forest. By virtue of their size and biomass contained in them, tropical ecosystems are dubbed as the great carbon (C) sink, and thus play a crucial role in our global carbon cycle. Moreover, these forests are home to millions of species of insects, thousands of species of animals, primary reservoir of freshwater, source of timber and non-timber forest products, medicinal plants and myriads of other uses benefiting the humankind.
Tropical rainforests are the most diverse and complex of all of the world’s ecosystems. Tropical rainforest contain the highest diversity of plants and animals, support the greatest mass living material per unit area, and maintain and uninterrupted pattern of growth throughout the year. The canopies of tropical rainforests are evergreen, constantly flowering and fruiting supplying the needs of myriads of vertebrates, insects, and microbes. Tropical rainforests are also referred to as “jungle” – which actually comes from the ancient Sanskrit of India, where the word “jangala” means a wilderness [39].
Importance of Tropical Rainforests
About half of the world’s drugs are based on plant products, many of these being from tropical forest species. In a complex environment like the forest, every plant is a potential food resource for the many animals in the community, and adaptation to such a competitive situation has led to the development of a range of chemical defenses in plants to deter the voracious grazers [39]. Many of these chemicals have direct and indirect impacts on animals (including human) physiology. What is poisonous or toxic or deterrent to one organism can be a potential source of cure to a specific disease or illness. The great arsenal of plant defense compound found in the tropical forest vegetation represents an immense resource for the pharmaceutical industry [39]. Natural defense mechanisms may also provide new pesticides (i.e. biological control) for agriculture and forestry that attack crops but that are much less harmful to the environment than artificial pesticides.
The role of forest in influencing the global climate has been supported by countless studies. By absorbing carbon dioxide from the atmosphere, tropical rainforests contain large reserves of carbon, locked in the organic materials that compose every part of the plant–from its root up to the canopy. These reserves of carbon (also referred to as carbon sink) are released back in the atmosphere during deforestation and burning.
The importance of tropical rainforest thus lies in its resources of wood, fibre, latex, toxins and drugs, and its contribution to the world’s hydrological cycle of water and atmospheric cycle of carbon and is oxygen provider.
Conservation Issues
Tropical rainforests are also among the most vulnerable and threatened habitats on Earth. Deforestation, which leads to tremendous loss of biodiversity is a very serious problem faced by tropical rain forests around the world. Since just 1950, the world’s population has more than doubled to more than 6 billion people, with the fastest population growth being in the tropics. Today, more than 3 billion people live in the tropics alone, much more than lived in the entire world in 1950s. To provide food, wood, fuel and resources for the world’s rapidly growing population, and to make rooms for the exploding tropical population, the world’s tropical rainforests are literally disappearing. Even with tropical deforestation at an all-time high, tropical hardwood prices continue to climb as world demand for tropical hardwoods continues to grow. A single teak log for example can now bring as much as RM 76,000. Annual world consumption of tropical hardwoods is now more than 250 million cubic metres.
Scientists estimate that until as recently as 10,000 years ago, the world had 24 million square kilometres of tropical rainforests. By 1950, we had a little less than 11.2 million square kilometres of rainforest. It was then being cut down at the rate of about 40,000 square kilometres to 60,000 square kilometres per year. Today we have less than 6.0 million square kilometres left, and we are clearing this remaining rainforest at the rate of 120,000 square kilometres per year, two to three times as rapidly as just a few decades ago.
Southeast Asia until recently has been the largest source of supply for tropical hardwoods, but that area will largely be depleted within the next few years. All of the primary forests in India, Sri Lanka, and Bangladesh are gone; patches are left in Malaysia, Indonesia and the Philippines. Ivory Coast’s forests are essentially non-existent. Nigeria’s forests have been decimated as well. As Asia’s and Africa’s tropical forests are depleted, consuming countries are turning increasing attention to Latin America and the Amazon, whose own rapidly growing population is also a source of pressure on the rainforests.
If the present rate of tropical deforestation continues, there will be nearly no tropical rainforests left in just 30 years. Instead of holding steady however, the rate of deforestation is actually predicted to increase even further. Scientists project that the rate of tropical deforestation will continue to increase for the next 10 to 15 years until there simply will not be enough forest left to sustain the rate of cutting.
As for the terrestrial ecosystem, there is the vast evergreen lowland dipterocarp forest, where the dominating vegetation is the dipterocarp from the plant family Dipterocarpaceae. Besides that there is the bamboo forest. Based on soil types, there is also the limestone forest where the soil is basically basic due to the occurrence of calcium carbonate forming limestone. The other soil type easily seen different from other types is the ultrabasic. Along the altitudinal gradient, forest below 500m is considered as lowland forest. Forest between 700m to 1,000m is the hill forest. Above that to a limit of about 1500m to 2000m is the submontane forest. A forest above this is considered as montane forest. The description is summarised in Fig. 32 [40].

7. TROPICAL LOWLAND FOREST OF MALAYSIA
Tropical lowland forest of Malaysia has the most luxuriant of all plant communities. It is lofty, dense, evergreen forest of 45m or taller, characterize by the large number of tree species which occur together. Gregarious dominance (consociations) are uncommon and usually two thirds or more of upper canopy tree are of species individually not contributing to more than 1% of the total number. This formation is conventionally regarded as having three layers: the top layer of individual or group of giant emergent trees, over a main stratum of 24-36m length and with smaller, shade-dwelling trees below that. Ground vegetation is often sparse and mainly of small trees; herbs are patchy. Some of the bigger trees have clear boles of 30m and reach 4.5m girth, and may be deciduous or semi-deciduous without affecting the evergreen nature of the canopy as a whole. Boles are usually almost cylindrical. Buttresses are common, cauliflory and ramiflory are also common features. Pinnate leaves are frequent; leaf blades of small size. Big, woody climbers, are mostly free hanging, and are frequent to abundant and sometimes also bole climbers. Shade and sun epiphytes are occasional to frequent. Bryophytes are rare. They occur in perhumid lowland climates where water stress is intermittent or absent, from sea-level to about 1200m on dry land. Two characteristics of a Malaysian tropical rainforest are featured in Fig. 33a and Fig. 33b– presence of epiphytes and climbers.


Animals inhabiting this tropical rainforest range from small size such as insects (ants, termites etc.) to large mammals (elephants, tembadau, orang utan etc.). Animals play important roles in the forest and vital to forest regeneration. Tiny insects, fungi, bacteria are critical decomposers that break down massive organic waste such as fallen trees and dead animals into nutrients that goes back to the soil. They are components of the nutrient cycle in the forest. Pollinations of flowers are carried out by an array of pollinators. These include birds, bats, insects. Seed dispersers again are the fruit/seed eaters such as elephant, primates, birds and small non-volant mammals and bats. An example of excellent seed dispersers is the hornbill. A species is seen in Fig. 34. It is clear that the functioning of the tropical forests of Malaysia, and the world over are much dependent on animals.

8. CANOPY
A characteristic of the tropical rainforest is the existence of several (2-3) layers of continuous and closed canopies. These canopies are formed by the dense foliage of trees (normally dipterocarps) at various height depending on maturity of the forest. In a climax forest, the first layer maybe of the height 50-60m and the second at about 30-40m.
According to Mitchell et al., 2002 [41], two of the greatest challenges facing natural world and are main concerns are loss of biodiversity and the impact of climate change. More than 50% global biodiversity may be in the canopy, much of it undiscovered and in the tropics; the canopy interfaces with the atmosphere and to date poorly understood, yet it directly affects climate changes and the carbon balance of the earth. As the forest destruction proceeds, window of opportunity to document the value of life it contains or its potentially crucial role in maintaining stability of our planet is closing.
Uses of canopy
Since ancient time, people had been harvesting products from the canopy. Honey of the wild bees is found mainly high up in the canopy or even emergents. There is a traditional belief that if honey is obtained from the menggaris or tualang tree (Koompasia excelsa), it is more potent and has been claimed to cure diseases such as cancer. In Malaysia wild honey could be found sold in traditional market called tamu, in Sabah and Sarawak (Fig. 35).

Fig. 35: Honey extracted from the wild bees nest deep in the tropical rainforest can be found sold in traditional market or tamu.
A characteristic of the lowland tropical forest of Malaysia is the occurrence of large number of epiphytes clinging itself to large straight bole or perching in between branches of dipterocarps or non-dipterocarps. These epiphytes are brought to this height by seed disperse such as birds and bats. They maintain their position up in the tree by growing and attaching their roots around the host plants. This position is important for epiphytes to survive because down at the forest floor there is little sunlight that could penetrate and, being sun lovers they are at a disadvantage position. Some epiphytes are parasitic, meaning not only they get support, epiphytes roots also suck some nutrient from the host tree. Most are non-parasitic and do not gain anything else from the host tree, except for the physical support. Examples of epiphytes are ferns (e.g. Birds nest ferns, stag horn ferns) and orchids.
Growing orchid has always been a hobby of many people in Asia. Scientifically many varieties had been developed. People are now looking for more rare orchids to grow. Sometimes, people collect orchids from the forest to sell in the nursery. These orchids may be of conservation importance. To avoid harvesting unsustainably from the forest, people could begin farming/ranching these kinds of orchid.
As there are plants growing at the canopy level, like the epiphytes, uses of these plants has not been researched into thoroughly. Some ferns from the canopy have now been grown in nursery and be sold as ornamentals. Other uses for plants and animals of the canopy, including for medicinal purposes need to be discovered.
Perhaps the most important is to understand how the canopy could be useful in combating the global changes. Since it is at the interface of atmosphere and lithosphere, perhaps foliage at the canopy level could make a difference to reduce effect of climatic change, example being increase in global temperature.
CONSERVATION OF THE TROPICAL RAINFOREST OF MALAYSIA
Occurrence of different types of vegetation is governed by several factors such energy available for the plants to grow. Tall trees of the tropical rainforest grow very well because of the availability of sunlight, being abundant along the equator. However, the lush evergreen dense canopy of the tropical rainforest hinders sunlight from reaching the forest floor. As such, certain trees have adapted themselves by having the ability to climb. These climbers, such the Gnetum have huge climbing trunks or roots. Some other groups like the rattan, have climbing trunks that have modifications with hooks that facilitate them to climb. These different plants available in the tropical rainforest created characteristics of the tropical rainforest morphologically and physiologically.
Comparing the different vegetation types between a tropical rainforest and a mangrove forest one could appreciate the clear differences.
In summary the tropical rainforests of Malaysia have these functions:
- Provide homes to people (e.g: the Penan of Sarawak) and animals (wildlife, including soil organisms such as worms, termites, ants, beetles, fungi)
- Provide nutrients to forest plants
- Protect forest soil (including from erosion) and its inhabitant
- Watersheds
- Produce oxygen as a by-product of photosynthesis
- Sequester carbon (trees of forest are carbon sinks)
- Climate modifier (moist from trees are kept within forest, reducing heat)
- Nutrient recycling process – decomposition of organic matters (dead trees and animals) turn back to nutrient by decomposers
- Provide food for animals and people
- Provide medicines to people
PEOPLE AND TROPICAL RAINFOREST
In Malaysia there are still indigenous people living in the forest. In Sabah there are Dusun communities living in and around the protected forest of the Crocker Range. There are several villagers and they have been occupying the villages since ancient time. In Sarawak, the Penans are still living in various parts of the forests. In Peninsular Malaysia some ethnics, such as those in Pahang and Ulu Kelantan are still living very near or in forests. Although, the Malaysian government had made effort to relocate them in villages, some still prefers to go back and live in the jungle. When there are events or the need to get medical services they will come out of the jungle to go to public gathering centres and clinics in the nearest village.
Despite the eagerness to achieve the status of a developed nation by 2020, Malaysia realised that some ethnics will not part from their birth place – the forest and the sea. And despite the thinking of the so called modern generation exemplified by James, 1999a [42] Kadazan Dusun ethnic, in his writing “Whither the Kadazandusun the next millennium” in 1999 there are still those that are comfortable in the forest of the Crocker Range, in Sabah. So are the Penans, choosing to inhabit the forests, just as their ancestors did, in the deep forest of Sarawak. And there are those that choose the sea, the Bajau Pelaut, swaying comfortably in their boat houses of the Sulawesi sea, at the east coast of Sabah. In Peninsular Malaysia, although many Orang Asli (the natives) has been given houses outside the forest fringes, they still go into the forest to harvest non-timber products for their daily use. Although, many urbanites will think that living in forest is not comfortable, it is not so for these ethnics.
PROTECTION OF SOIL AGAINST EROSION
Soil consists of organic and inorganic matters. Organic matters present in soil are living things such as worms, insects, bacteria and fungi. Inorganic matters are the rocks, sand, metals. Rocks, stones, pebbles disintegrate to become smaller particles that made soil. Besides soil particles, there are nutrients that are essential for plant growth. Soil also keeps water. Roots of plants absorb water and nutrients from soil to make their own food through the photosynthesis process. Besides plants, soil also is habitat for many kinds of living things like worms, sow bugs, millipedes and insects. These organisms make galleries and burrows and live under the soil surface. Some also live on decomposing leave litter that cover soil. Contrary to the belief, tropical forest soil is rather poor in nutrients, because much of the nutrients are being absorbed at a fast rate by the huge trees. Decomposition of dead plants and animals are carried out by tiny organisms such as bacteria, fungi, worms and tiny insects (ants, termites, beetles and others). Bigger organisms such as millipedes, and isopods are also decomposers. They have mechanisms to digest dead organisms and turn them to nutrients that go back to the soil. As long as forest floor is covered by the canopy of the forest, most of the top rich soil will be retain. When the canopy are gone (due to cutting of trees), the soil will be exposed to sunshine (killing some of the decomposers) and rainfall that erodes the topsoil. Erosion of top soil will make forest soil poorer and the eroded soil particles will be brought to the rivers causing shallowing of the river bed. This may cause flooding whenever there is heavy rainfall, where the river could not retain all the water and extra water will overflow on to the river banks.
Besides that, sediment from rivers in the forest is a kind of pollution and will flow other bigger rivers whose water may be used as a source for domestic uses in towns. Eventually this sediment will flow into the sea causing siltation and pollution to the sea.
FOREST AS WATERSHEDS
Forests are watersheds. This is because, rain water that falls into the forest are absorbed and kept within the forest soil. The water level in the forest is maintained relatively high and stable by the sponging effect of the root system of the forest trees. The humidity in the forest and coolness prevent dryness and thus maintained the wet condition of the watershed. The stable ground water level of the forest soil is much determined by this root system. Water in the ground of a forest will eventually flow into lower river bed and provide good water to the river. Seen in Fig.36 is Sg. Kimanis that provide clean water to the eastern coast region of Sabah.

HOME FOR ANIMALS
It is a known fact that the tropical rainforest of Malaysia is one of the oldest in the world. Evolutionary processes have been going on undisturbed for a long time, until the 40s and 50s, when colonisation of Malaya and Borneo, carry with it the toll of deforestation for logs and for agricultural purposes. Thereafter, even after Malaysia gained independence, there has been an extensive use of the forest to plant more agricultural crops to generate income for the socio-economic development of the people. But the wildlife are still in need for homes. Thus, presently large tracts of forest are being set aside as permanent forest reserve to provide habitats for wildlife and others. Some tracts are large enough and may contain representatives of most animal groups. An example is the Taman Negara (National Parks) that spans across three states in Peninsular Malaysia, Kelantan, Terengganu and Pahang. Smaller ones are state parks such as Endau Rompin in Johor, Wang Mu and Wang Kelian in Perlis, Royal Belum Park in Perak and Gunung Stong in Kelantan. In Sabah, the famous Danum Valley is an excellent example of lowland forest conservation area. Wildlife of these parks include the largest mammal in Malaysia, the Asian elephant (Elephas maximus).
9. HILL FOREST
Taking into account altitude, tropical forest at the height of 500-1000m is considered as hill forest. Thus, as an example, the Crocker Range of Sabah could partially be classified as Hill forest, as before it culminate at about 1100m, major portions is 500m asl (above sea level).

10. SUBMONTANE FOREST
Submontane (or tropical lower montane) forest may be classified as those between 1000 to 2000m. These forests are normally ever-green but shorter in height, the canopy at about 15-33m (Table 6). Gunung Mas forest in Sabah is a good example of a submontane forest, as well as part of Gunung Trus Madi. In the Peninsular Malaysia, the top part of Gunung Ledang, Gunung Tahan and Gunung Brinchang are examples of submontane forest. To compare vegetation characteristics of tropical lowland, tropical lower montane and tropical upper montane forests, Table 6 is given. Figure 38 illustrates the vegetation of Gunung Emas, a submontane forest of Sabah.
Table 6: Characteristics of structure and physiognomy used to define the principal montane forest formations [40].
| Formations and characteristics | tropical lowland evergreen rain forest | tropical lower montane rain forest | tropical upper montane rain forest |
| Canopy height | 24-45m | 15-33m | 1.5-18m |
| Emergent trees | characteristics, to 60(80)m tall | often absent, to 37m tall | usually absent, to 26m tall |
| Pinnate leaves | frequent | rare | very rare |
| Principle leaf size class of Woody plants | mesophyll | mesophyll | microphyll |
| Buttresses | usually frequent and large | uncommon, small | usually absent |
| Cauliflory | frequent | rare | absent |
| Big woody climbers | abundant | usually none | none |
| Bole climbers | often abundant | frequent to abundant | very few |
| Vascular epiphyes | frequent | abundant | frequent |
| Non-vascular epiphytes | occasional | occasional to abundant | often abundant |
|
Fig. 38: The submontane forest of Gunung Emas. |
11. MONTANE FOREST
Tropical rainforests are not confined to the lowlands of the tropics but are also an important feature of the more mountainous areas. Montane forest is also referred to as cloud or mossy forest. The structure and composition of montane forest vary with altitude due to the alterations in climate. Large lianas or the woody climbing vines that are common in lowland tropical rainforest are less likely to be found in montane forests. Montane forest is generally dominated by ferns, mosses, liverworts, orchids, and bromeliads among others. There are still trees found in these zone however, their physical appearance is much different from their close relative in the lower elevations. Trunk of montane forest trees are much shorter and twisted due to continuous exposure to strong winds. In Malaysia, montane forest can be found in Mount Kinabalu in Sabah. Observing from the foot of the mountain, at the lower altitude, for example at Poring, at 300-500m asl, the dominant vegetation are similar to lowland forest. There are big trees of the dipterocarp family and legumes. The main trunk or boles are straight with no branches until it reaches some height perhaps at 30-40m. Then there is the huge canopy. To support this bulk there is the huge buttress system. Buttresses are part of the root system that flanges above the ground level. This structure gives support for the huge dipterocarp trees. Since, canopies of these huge trees are extensive, it forms a closed layer, preventing much sunrays from falling to the forest floor. This then causes some plants to develop thin rope like trunks or branches or roots (lianas) that climb the straight bole of dipterocarp to reach for the sunlight at the top. Besides lianas we have other plants that grow on the dipterocarp and we call them epiphytes.
As we climb Mount Kinabalu at about 1000m to 2000m asl, bigger trees tend to be of shorter height. There are still buttresses but of smaller size. Lianas are still abundant but woody climber is no longer seen. At 3000m asl, we begin to notice that trees are stunted, with gnarled trunks. Leaves of these trees are adapted to the cooler temperature and windy climate. The wet and cool ambience provided an ideal condition for mosses, and ferns to flourish. Further up the mountain at about 3800m asl, no trees are found just mosses. Figure 39 shows a view of Kinabalu with submontane vegetation at the bottom part of the photographs and the bare summit at about 4010m asl.
The submontane and montane forests are more important as watershed areas as most rains fall on the mountains. The montane forest are the first line “sponges” for retaining water in their thick litter, and release water according to gradient to the lower ground. The north-east, south-west Crocker Range on the west coast of Sabah, is a highland that acts as an important water shed for areas on the west and interior [43].

At the summit there is no apparent vegetation but mosses and lichen could be found in between crevices of the rocks. Figure 40 shows the summit. Some iconic plant species of the submontane and montane forests are pitcher’s plants (Nepenthes), Rhododendron, and members of the berangan (oak) or the mempening family (Fagaceae), kelat family (Myrtaceae), Ericaceae and medang family (Lauraceae) which are more predominant on the higher altitude habitats of Malaysia. A good description of vegetation at the different elevations of Mount Kinabalu can be found in Wong and Phillipps, 1999 [44].

12. BAMBOO FOREST
Bamboo forest is a unique type in a tropical rainforest ecosystem and is not as common as the mixed-dipterocarp forest. In Malaysia, bamboo forest or “Bamboo-Schima” forest is found in the contiguous Belum and Temenggor Forest Reserves – being one of the oldest vegetation (approximately 130 million years old). It is different from the remaining tropical rainforest of Malaysia as the Bamboo-Schima forest is more associated to the northern monsoonal Burmese-Thai vegetation. This forest is home to unique animals such the Asian elephant, Sumatran rhinoceros, Malayan tiger, Malayan gaur, leopard, and tapir among others.
Bamboos grow in either pure stand or mixed with trees. They are water tolerant, light demanding and fast growing. Develop steadily on flat land, hills and along streams with as short rotation of 2-4 years. They are normally found growing wild or planted by people along landslide and river banks, which is useful in preventing further damage to soil especially erosion. Bamboo shows characteristically clumped growth. For Schizostachyum grande, an elegant species becomes abundant in seriously disturbed lowland evergreen rain forest in the mountains at 600-800m elevation.
Many plant grow by tufting habit, as a result of a basal sympodial branching, and this is common among monocotyledons as a whole and also found for example in gingers, bamboos, and bananas. The apical leaf tuft may collect falling detritus, claimed to provide for the source of humus and nutrient for the tree.
Bamboo loves sunlight. In the Malaysian forest, sometimes, you could come across pure stand of bamboos. One good example is in Poring, a tourist destination at the foot of Mount Kinabalu (Fig. 42). There bamboo group is so dominant that the site is called Poring, which in the Dusun language means Bamboo. The local term for bamboo is bambu or buluh, and characteristically it has long non-branching stems. Normally bamboo grows in clumped form, and as the individual tree grows upward, more young shoots will develop at the base [45].
Bamboo Uses
Bamboo is now recognised as one of the common NTFPs (non-timber forest products). Another example of NTFP is the rattan. Bamboo and rattan could be useful to the furniture industry. Beside that they are useful for the art and craft industry. During olden days bamboo are useful in making flooring and walls for wooden house. More recent, the use of bamboo is extended to providing framework for scaffolding. It is light but strong. People who had concession over bird nests will harvest the nests sited at great height on wall and ceilings of caves like Gua Gomantong and Gua Madai in Sabah using such scaffolding structures. Modern day uses of bamboo in the homes are illustrated in Fig. 43a and Fig. 43b. Being one of the leading countries where bamboo is extensively distributed, many more bamboo products are used in China such as handicraft, utility items, furniture, parquet [46].


In Malaysia bamboo shoot are eaten by several groups of ethnics like the Chinese and Malay, as well as Dusun, Bajau, Iban, Jakun and many others. Bamboo has also been made into chopsticks. In Malaysia satay a local favourite and popular were skewed on to stick made from mid rib of coconut leaves. Now most satay are skewed on to bamboo sticks. A local glutinous rice cake (lemang), are cooked with coconut milk in bamboo, over slow fire. Recent research looks at bamboo for making paper.
13. HEATH FOREST
Another rather unique ecosystem of the dryland forest is the heath or kerangas forest. This forest type is normally found on poor soil. As such the vegetations are stunted. This creates an open low canopy forest with sparsely distributed large trees. The well-known Maliau Basin of Sabah has large acreage of kerangas forest on the southern and western ridge. An expedition held in 1996 reported in a monograph Maryati et al., 1998, [47] described the uniqueness of this ecosystem. A typical view of this ecosystem is shown in Fig 44. This photo is taken from Bako National Park of Sarawak, another well-known heath forest.

In Maliau Basin, Widodo & Maryati, 1998 [48] noted the abundance of ant plants in the heath forest. As seen in Fig. 45, ant plants are epiphytes that grow on the main trunk of stunted trees. The species normally found in this ecosystem are from the genera Myrmecodia and Hydnophytum of the Rubiaceae (coffee) family. Ants build galleries in the tubers of the ant plants, and make them their nests. Ants protect the growing shoots of ant plants and brought minerals from soil as they return to the nests that they make in the galleries. In return, ants are provided with homes and food in the form of food bodies as described by Widodo and Maryati, 1998 [48]. In this photo (Fig. 45) taken from Bako, ant plants are shown growing on a stunted tree trunk. Similar ant plant can be bought from local street markets in Sabah and in Fig. 46 is an ant plant open up to show galleries built by the ants.


Another typical plant group found in poor soil is the pitcher plants (Nepenthes). In Maliau Basin some eight species had been recorded: N. mirabilis, N. hirsuta, N. lowii, N. reinwardtiana, N. stenophylla, N. tentaculata, N. veitchii and the natural hybrid N. stenophylla X veitchii [49].
As for animal life, due to the poor vegetation available in kerangas forest, diversity is rather low. Earlier report from Maliau reported on the poor diversity of mammals (four species of tree shrews), birds (182 species), frogs (25 species) and reptiles (9 species of snakes). As the water of Maliau rivers is acidic, only four species of fish were recorded [50]. In later years Hazebroek et al., 2004 [49] gives a richer picture of animal life (86 species of mammals; 270 species of birds, 46 species of frogs and toads). Bako National Park comprises of a variety of ecosystems, varying from open shrub land to dense heath forest to lush tropical forest and the animal diversity was recorded at 37 species of mammals, 184 species of birds and 24 species of reptiles. And since the mangrove is one of the ecosystem in Bako, the proboscis monkey (Nasalis larvatus), endemic to the island of Borneo is also found here.
Heath forest is important ecologically as it is a different forest type that flourishes despite the poor soil foundation. As such it harbours an array of plants and animals some of which are not common elsewhere, such as the Nepenthes and ant plants. The adaptability of some of these organisms to such poor soil condition can only be illustrated in a heath forest.
14. LIMESTONE FOREST
In Malaysia another peculiar ecosystem is the limestone forest. The soil generated at this ecosystem is normally rich with calcium carbonate. They are normally outcrops of limestone that form a system of caves and streams (Fig. 47). Famous limestone cave in Malaysia are the Mulu and Niah caves in Sarawak. Gunung Mulu is reported to have 1,500 species of flowering plants (10% of Malaysian diversity), 170 species of orchids, 10 types of pitcher plants, 67 species of mammals, 267 species of bird, 74 species of frogs and 47 species of fishes [56]. Besides the rich biodiversity, Mulu Caves also has the world’s largest natural rock chamber at 600m long, 450m wide and 100m high. In Sabah, Gomantong Caves situated in Sandakan is historically important and is still is involved with production of birds nest. The indigenous people who has been living near Gomantong Caves and in Madai Caves has legal claim to the nests of the swiftlet built in these caves. There is even an enactment that protect the right of the indigenous people. During the breeding season, they will harvest the nests using scaffoldings made of long ladders made of bamboo. Similar practice is carried out in Niah Caves in Sarawak.

It is interesting to note that besides swift another dominant occupant of the limestone caves is the bat. Every day just after sunset there will a stream of bats flying out of the cave and a steady stream of swift-lets entering the cave. The next day just as the sun is rising, the opposite will occur, the flying in of bats and the flying out of bats. This maximises the use of the caves as roosting site for two different groups of organisms.
Vegetation collected from limestone areas included palms[51], gingers (23 species from 12 genera from a limestone ridge of Tabin with Elettariopsis sp. being a limestone specialist [52], seed plants (17 new species for record of Tabin Wildlife Reserve [53] and herbaceous vegetation. There were 16 unique limestone species with poor Zingiberaceae species but a relatively high Araceae diversity. The total, reached 86 species (16% ferns and fern allies, 35 dicotyledons and 49 monocots, and two thirds of monocots were Araceae [54]. All limestone vegetation grows on very poor soils as the nutrients are constantly washed away by the rainfall. Many plant species grow in the crevices made by the weathering and erosion of the limestone over time.
For the fauna record, Schilthuizen & Vermeulen 2003 [55] listed 64 secies of land snail, similar to other Lahad Datu-Segama malacofauna. One endemic species was found, Diaphera wilfordii wilfordii. Another in abundance was Cyclophorus kinabaluensis. Diplommatina oedogaster recorded its second location in Gomantong Cave, and this call for its uniqueness and the need to protect. As for butterflies, Mohd. Fairus et al., 2003 [56] updated the species list to 310 species.
Price, 2005 [57] stated that Peninsular Malaysia is about 131,794sq km, and one third of the surface is being covered with limestone. In Malaysia as a whole less than 1% is limestone hills. About 0.3% north of Peninsular Malaysia have many lime stones hills. The southern-most limestone outcrop is Batu Caves, and in fact, it is the southern-most karst tower in Asian mainland.
Limestones vary in age, in Peninsular Malaysia they range from Ordivician (450million years old) through the Carboniferous and Permian in the Upper Triassic (220 million years). Most occur within the Permian. Older limestones are darker in colour. Older limestones give rise to more or less continuous ranges of hills without marked vertical cliffs such as the limestone of the Setul formation in the northwest of Perlis, that extended into Thailand. Younger limestone produce more isolated hills with notably higher vertical or even overhanging cliffs. The limestone usually outcrops as aligned ridge or towers dominating the plain. A good example is the Kinta Valley in Perak. Hills are often small, the largest has a diametre of a few kilometres but most are only a few hundred metres across. These characteristic hills are often isolated and have sheer vertical cliffs with distinctive flora of semi-drought vegetation compared to the normal rain forest the residual soils on the outcrops are meager and support only thin scrubby vegetation.
Another limestone area in Malaysia that has been explored in 2001 and reported in 2005 is one of the bigger limestone outcrops in the Kinta Valley, the Lanno Limestone [58]. It is an isolated limestone mountain densely overgrown with primary forest. During a three weeks survey, 32 caves were documented and 12 km passageways surveyed. Puncak Caves holds one of western Malaysia largest chambers, Lanno Summit Chamber, 60m wide and 180m long and located directly below the peak of the mountain.
According to Steiner 2005 [59], cave, by definition is underground, that is dark, devoid of sunlight. To sustain life, different sources of energy tapped-guano system (versus ecosystem) is linked to the surface systems of bats. It feeds outside and return to caves to roost. Number of bats is huge as can be seen in the Deer Caves at Gunung Mulu National Park, in Sarawak at 600,000 [60]. Sarawak has several extensive limestone formations, two best known being Mulu and Bau. Rivers that run through limestone are characterized by hard (high calcium content) alkaline water (pH > 7). The left and right tributaries of Sarawak River (Sarawak Kiri and Sarawak Kanan) flow through Bau Limestone Region and this contribute to diversity of fish species and have higher argillaceous content than young ones and this support sufficient residual soil to sustain a fairly abundant vegetation.
EFFORTS IN CONSERVATION OF TROPICAL RAINFOREST
Malaysia has one of the oldest tropical rainforest in the world. In addition, the tropical rainforest of Malaysia is very rich with plant diversity. In turn, this huge plant diversity harbours a rich animal diversity. Along with the naturally occurring evolution through millions of years, the tropical rainforest of Malaysia has high representative of global plant and animal groups. As Malaysia advances in time, especially after she gained her independence in 1957, the socio-economic progress is rapidly taking place. As with other parts of the world any socio-economic development will affect conservation. Once upon a time forested, certain percentage of this forested areas have to be developed into public amenities – roads, schools, administrative buildings, hospitals, universities. New township flourishes, residential facilities have to be provided for people. As population increases, more lands are converted for several land-uses including for cultivation of economic crops, to generate income for the nation for development. Despite the rapid development the Malaysian government is aware of the consequences of land use changes, locally as well as globally.
Conservation of ecosystem in Malaysia has always been high in the government agenda. In line with global trend, Malaysia has been putting aside several areas as protected areas (PAs).To get a better idea of the size of the various ecosystem that are protected in Malaysia, Table 7 is given. In the table, Mount Kinabalu in Sabah and the national park – Taman Negara in Peninsular Malaysia (established in three states in Peninsular Malaysia – Kelantan, Terengganu and Pahang) are listed.
Table 7: Some of the Malaysian protected ecosystems [4].
| Protected Area55,400Protected Area | Size (hectares) | Type of Ecosystem | Important Plants | Important Animals |
| Bako National Park (Sarawak) | 2,742 | Beach forest, mangrove, swamp, mixed dipterocarp, kerangas | Nearly every plant in Borneo is found here | Proboscis monkey, silver-leaf monkeys, long-tailed macaque. Hornbill |
| Crocker Range Park (Sabah) | Lower-montane forest, Lowland ldipteropcarp forest | Rafflesia, orchids, | Orangutan, gibbons, macaques, tarsier, civet cats, hornbill | |
| Enda Rompin National Park (Johor) | 48,905 | Mixed diptarocarp, heath forest, riparian and riverine ecosystems, | Palms, ferns, orchids, | Elephant, monkey, tiger, leopard cat, civet, porcupine, |
| Gunung Mulu National Park (Sarawak) | 55,400 | Limestone karst ecosystem, mixed dipterocarp forest, submontane forest, riverine ecosystem | Orchids, pitcher plants, ferns, mosses | Primates, fish, frogs, insects, bat |
| Kenong Rimba Park (Pahang) | 12,800 | Mixed dipterocarp forest, riverine, cave ecosystem | Orchids, pokok Ara, parasitic plants | Mouse deer, porcupine, elephants, birds, fish |
| Kinabalu National Park (Sabah) | 434,300 | Montane, sub-montane, hill-dipterocarp, lowland mixed-dipterocarp | Orchids, ferns, mosses, pitcher plants, rafflesia | Birds, primates, birds, |
| Niah National Park (Sarawak) | 3,120 | Limestone karst | Birds, bats, Raja Brooke butterflies, | |
| Rantau Abang Turtle Hatchery (Terengganu) | Marine ecosystem | Leatherback turtle | ||
| Taman Negara (Pahang, Terengganu, Kelantan) | 434,300 | Submontane forest, Mixed dipterocarp forest, lowland tropical rainforest | >10,000 species of plants | Tapir, elephant, tiger, leopard |
| Tunku Abdul Rahman National Park (Sabah) | 4,929 | Lowland dipterocarp forest, Island ecosystem, marine ecosystem |
Challenges in conserving the tropical rainforest of Malaysia are many. Firstly, people have to understand their role as the steward of mother earth. And this must come in different forms of effort including raising public awareness and environmental education. Secondly, in rapidly developing Malaysia we have to be aware of the impacts of the physical and infrastructural development on the natural resources of the nation. Long term ecological research has to be done to study on how to minimise the impacts. Periodic monitoring is also critical. In forest conservation, management of logged over forest is of outmost importance as more and more forested areas are being developed for socio-economic development. Sustainability of forest depends very much on harvesting techniques. Research and innovation are needed to learn on how to maximise the potential of forest, and at the same time conserving it.
REFERENCES
- Odum, E.P. 1983 Basic ecology Saunders College
- Convention on Biological Diversity 1991 Canada
- Whitmore, T.C. 1998 An introduction to tropical rain forests 2nd edition Oxford university Press pp282
- MOSTI 1997 Assessment of Biological Diversity in Malaysia Ministry of Science, Technology and the Environment
- Earthscan 2003 Earth Trends Country Profile : Malaysia Earthscan
- Milne, D.H. 1995 Marine life and the sea Belmont, CA:Wansworth Publ.
- Karleskint, G., Turner, R. Small, J.W. 2010 Introduction to marine biology Belmont CA: Brook/Cole
- Trono, Jr. G.C. 1999 Diversity of sea weed flora of the Philippines and its utilization Hydrobiologia 398/399 : 1-
- Dahlan Taha, Zulkefli Mokhtar., Suhaili Rosli. & Noradil@Mohd. Adli Parsada 2007 National Tree Planting Programme for Coastline Protection under the 9th Malaysia Plan by Forestry department of Peninsula Malaysia In: Kobayashi, K. & Maisarah Ali (Eds) Proceedings of International seminar on Wetlands and Sustainability, Johor Baru, Johor, Malaysia 361-376
- Suhaili Rosli 2010 Cabaran dalam pemuliharaan hutan pesisiran pantai : pengalaman JPSM Paper delivered at National Seminar on Coastal Morphology 2010 : The muddy coast of Malaysia, organized by National Hydraulic Research Institute of Malaysia, Serdang, Selangor (17 June 2010)
- Vaz, J. 1997 The Kinabatangan floodplain : An introduction
- Lee Shan Khee 2002 Human-elephant conflict in Lower Kinabatangan Postgraduate Dissertation Abstracts 1999-2006, ITBC, Universiti Malaysia Sabah pp: 51-
- Nurzhafarina Othman, Maryati Mohamed, Abd. Hamid Ahmad, Nathan, S. Pierson, H.T. & Goossens, B. 2008 A preliminary study on the morphometrics of the Bornean Elephant. Journal of Tropical Biology And Conservation 4(1) : 109-113
- Rashid Saburi 2006 Conservation of Orang utan in the lower Kinabatangan floodplain, Sabah, Malaysia Postgraduate Dissertation Abstracts 1999-2006, ITBC, Universiti Malaysia Sabah pp: 55-
- Gisil, J., Suleiman, M., Maripa, R.D. & Mohd., Fairus, J. 2003 Additions to the vascular plants from Lower Kinabatangan In : Maryati Mohamed, Takano, A., Goosens, B. & Indran, R. 2003 Lower Kinabatangan Scientific Expedition 2002 Universiti Malaysia Sabah. Pp:77-82
- Azmi, R. 1998 Natural vegetation of the Kinabatangan floodplain Part I : An introduction to its natural vegetation including a preliminary plant checklist of the region WWF Malaysia, Kuala
- Sahana H. & Maryati Mohamed 2006 reseraches in wetland : Lower Kinabatngan Floodplain, Sabah, Malaysia paper presented in Asian Wetland Intrenational Seminar, Vietnam. 15pp
- Mastaller, M. 1997 Mangroves : The Forgotten Forest Between land and Sea Tropical Press Sdn. Bhd.
- Yusoff, F.M. & Gopinath, N. 1995 The status of inland fisheries in Malaysia In: T. Petr. M. Morris (Eds) Indo-Pacific Fishery Commission, pp225-239 FAO Report No. 512 Supplement
- FRIM-UNDP/GEF Peat Swamp Forest Project 2004 Black water jewel – South East Pahang Peat Swamp Forest FRIM-UNDP/GEF Peat Swamp Forest Project and the Pahang Forestry Department in Collaboration with Wetlands International Malaysia, 58pp
- Ongkili , M.J. 2005 Keynote Adress : Conservation and management of peat swamp forests and other wetlands in Sabah – issues and challenges In : Kugan, F. & Chey, V.K (eds.) Conservation and Management of peat swamp forests and other wetlands in Sabah : Issues and challenges Proceeding of the 9th. Sabah Inter-agency Tropical Ecosystem (SITE) Research Seminar Sabah Forestry Department pp: 11-17
- .Atack, K. 2006 A field guide to the fishes of Kuching River Natural History Publication (Borneo) 200pp
- Kugan, F. & Chey, V.K (eds.) 2005 Conservation and Management of peat swamp forests and other wetlands in Sabah : Issues and challenges Proceeding of the 9th. Sabah Inter-agency Tropical Ecosystem (SITE) Research Seminar Sabah Forestry Department 222pp.
- Rashid, A.S., Efranshah & Abdul Rahim Nik 2005 Development of an integrated plan for Klias Peat Swamp Forest and associated wetlands : Lessons learned in promoting consultative process In : Kugan, F. & Chey, V.K (eds.) 2005 Conservation and Management of peat swamp forests and other wetlands in Sabah : Issues and challenges Proceeding of the 9th. Sabah Inter-agency Tropical Ecosystem (SITE) Research Seminar Sabah Forestry Department pp: 2-
- Sato, H. & Anton, A. 2000 Freshwater periphytic algae of rivers in the Klias and Binsulok Forest reserves, Sabah In : Maryati Mohamed, Mashitah Yusoff & Unchi, S. (eds) Klias-Binsulok Scientific Expedition University Malaysia Sabah pp: 13-
- Berhaman, A. & Sugau, J.B. 2005 A checklist of flora of the Klias-Binsulok Peat Swamp Forests, Sabah, Malaysia In : Maryati Mohamed, Mashitah Yusoff & Unchi, S. (eds) Klias-Binsulok Scientific Expedition University Malaysia Sabah pp:19-29
- Ong, R.C.,, Nilus, G.H., Sugau, J.B. & Lim, S.P. 1998 Forest of Bukau – Api-Api River area, Klias Peninsula : A botanical assessment and conservation perspective. Forest Research Centre, Sabah Forestry Department, Sandakan, A report for WWF
- Takahashi, A. & Berhaman, A. 2000 Nepenthes in the peat swamp forest of Klias Forest Reserve In : Maryati Mohamed, Mashitah Yusoff & Unchi, S. (eds) Klias-Binsulok Scientific Expedition University Malaysia Sabah pp: 29-34
- Nishida, S. & Maryati Mohamed 2000 Preliminary study of the cuticular features in eleven angiosperm species from Klias and Binsulok, Sabah In : Maryati Mohamed, Mashitah Yusoff & Unchi, S. (eds) Klias-Binsulok Scientific Expedition University Malaysia Sabah pp: 35-42
- Mohd. Fairus Jalil, Nordin Hj. Wahid & Maryati Mohamed 2000 A preliminary assessment of the butterfly fauna of a peat swamp forest In : Maryati Mohamed, Mashitah Yusoff & Unchi, S. (eds) Klias-Binsulok Scientific Expedition University Malaysia Sabah pp: 43-48
- Corbet, A.S. & Pendlebury, H.M. 1992 The butterflies of Malay Peninsula, 4th edition Revised by J.N. Eliot. Malaysian Nature Society, Kuala Lumpur
- Mohd. Nadzri Ishak, Maryati Mohamed, Arifin Ag. Ali, Guraim Gueh & Dionysius Laison 2000 Ichthyofauna of Klias and Binsulok – A survey and its significance In : Maryati Mohamed, Mashitah Yusoff & Unchi, S. (eds) Klias-Binsulok Scientific Expedition University Malaysia Sabah pp: 49-
- Maryati Mohamed, Mashitah Yusoff & Unchi, S. 2000a Klias-Binsulok Scientific Expedition Universiti Malaysia Sabah
- Yasuma, S., Arlyna Abdullah and Bernard, H. 2000 Notes on mammals of Klias and Binsulok Forest Reserves, Sabah In : Maryati Mohamed, Mashitah Yusoff & Unchi, S. (eds) Klias-Binsulok Scientific Expedition University Malaysia Sabah pp: 69-74
- Dalimin, M.N. & Zulistiana, Z. 2005 Physical environment of the Klias-Binsulok Forest Reserve, Sabah In : Maryati Mohamed, Mashitah Yusoff & Unchi, S. 2005 Klias-Binsulok Scientific Expedition university Malaysia Sabah pp:7-12
- Greer, T., Jessen, O., Matunjau, C. & Yap, S.F. 2005a Hydrological assessment of the Klias Forest Reserve In : Kugan, F. & Chey, V.K (eds.) 2005 Conservation and Management of peat swamp forests and other wetlands in Sabah : Issues and challenges Proceeding of the 9th. Sabah Inter-agency Tropical Ecosystem (SITE) Research Seminar Sabah Forestry Department pp: 39-
- Greer, T., Jessen, O., Matunjau, C. & Yap, S.F. 2005b Principles of developing a water management strategy for a peat deposit based on Klias Forest Reserve and surrounding areas In : Kugan, F. & Chey, V.K (eds.) 2005 Conservation and Management of peat swamp forests and other wetlands in Sabah : Issues and challenges Proceeding of the 9th. Sabah Inter-agency Tropical Ecosystem (SITE) Research Seminar Sabah Forestry Department pp: 50-
- Deratil bin Boaklan, Jenny, N.L.L. & Malangkig, E. 2005 Capacity evaluation of the peatland in Kuala Penyu and Beaufort for sustainable agricultural development In : Kugan, F. & Chey, V.K (eds.) 2005 Conservation and Management of peat swamp forests and other wetlands in Sabah : Issues and challenges Proceeding of the 9th. Sabah Inter-agency Tropical Ecosystem (SITE) Research Seminar Sabah Forestry Department pp: 66-
- Moore, R. D. 2008 Emerging threats to tropical forests Copeia (2003)3: 727-
- Whitmore, T.C. 1984 Tropical rainforest of the far east (2nd ed.) Clarendon Press, Oxford pp: …
- Mitchell, A.W., Secoy, K. & Jackson, T. (Eds.) 2002 The global canopy handbook : Techniques of access and study in the forest roof Global Canopy Programme 248pp
- James, G. 1999 Whither the Kadzandusun the next millennium Kadazandusun Cultural
- Kusano, T. 2004 Conservation of Crocker Range Park In : Maryati M., Zulhazman, H. Tachi, T. & Nais, J. 2004 Crocker Range Scientific Expedition 2002 Universiti Malaysia Sabah pp: ix
- Wong, K.M. & Phillipps, A. 1996 Kinabalu : Summit of Borneo The Sabah Society In association with The Sabah
- Dranfield, S. 1992 The bamboo of Sabah, Sabah forest records no. 14
- Wang, K.L., Hong, L.T. & Rao, V.R. 2005 Bamboo resources and traditional culture in Xishuangbanna, Yunnan, Southwest China IPGRI-APO, Serdang, Malaysia
- Maryati Mohamed, Sinun, W., Anton, A., Dalimin, M.N & Ahmad, A.H. (eds) 1998 Maliau Basin Scientific Expedition Universiti Malaysia Sabah pp: 119-132
- Widodo, E.S. & Maryati Mohamed 1998 The epiphytic ant-plants (Family Rubiaceae) of the Maliau Basin In : Maryati Mohamed, Sinun, W., Anton., Dalimin, M.N. & Ahmad, A.H. (eds) Maliau Basin Scientifc Expedition Universiti Malaysia Sabah pp: 113-119
- Hazebroek, H.P., tg. Zainal Adlin & Waidi Sinun 2004 Maliau Basin : Sabah’s Lost World Natural History Publication (Borneo) Sdn. Bhd. Kota Kinabalu
- Smith, K.M., Samat, A., Hee, T. S & Tong, T.H 1998 The fish and crustacean fauna of the Maliau Basin In : Maryati Mohamed, Sinun, W., Anton., Dalimin, M.N. & Ahmad, A.H. (eds) Maliau Basin Scientifc Expedition Universiti Malaysia Sabah pp: 119
- Andersen, J., Boje, K.K. & Borchsenius F. 2003 The palm flora of Tabin Wildlife Reserve In : Maryati Mohamed, Schulthuizen, M. & Andau, M. (eds) Tabin Limestone Scientific Expedition 2000 Universiti Malaysia Sabah pp:41-48
- Gobilik, J., Magintan, D., Poulsen, A.D. & Mashitah Yussoff 2003 Gingers at the Dagat Limestone Ridge, tabin Wildlife Reserve In : Maryati Mohamed, Schulthuizen, M. & Andau, M. (eds) Tabin Limestone Scientific Expedition 2000 Universiti Malaysia Sabah pp:49-60
- Gisil, J. & Suleiman, M. 2003 Additions to the seed plants of Tabin Wildlife Reserve In: Maryati Mohamed, Schulthuizen, M. & Andau, M. (eds) Tabin Limestone Scientific Expedition 2000 Universiti Malaysia Sabah pp: 61-63
- Kjeldsen, K.H. 2003 The herbaceous vegetation of Limestone in tabin Wildlife Reserve In: Maryati Mohamed, Schulthuizen, M. & Andau, M. (eds) Tabin Limestone Scientific Expedition 2000 Universiti Malaysia Sabah pp: 77-92
- Schilhuizen, M. 2003 The land snails of the Tabin Limestone Hill In: Maryati Mohamed, Schulthuizen, M. & Andau, M. (eds) Tabin Limestone Scientific Expedition 2000 Universiti Malaysia Sabah pp: 93-98
- Mohd Fairus Jalil, Nakanishi, A., Maryati Mohamed & Nordin Wahid 2003 Updates and revisional notes on the butterflies of Tabin Wildlife Reserve In: Maryati Mohamed, Schilthuizen, M. & Andau, M. (eds) Tabin Limestone Scientific Expedition 2000 Universiti Malaysia Sabah pp: 99-108
- Price, W.P. (Maryati Mohamed – translator) 2003 Insects ecology Dewan Bahasa dan Pustaka pp:30
- Geyer, E., Schmidt, F. & Jeutter, P. 2005 Speleological expedition Gunung Lanno Verein fur Hohlenkunde in Obersteier, Bad Mitterndorf, Austria
- Steiner, H. 2005 Cave fauna of Malaysia In: Geyer, E., Schmidt, F. & Jeutter, P. 2005 Speleological expedition Gunung Lanno Verein fur Hohlenkunde in Obersteier, Bad Mitterndorf, Austria
- Hall L. 1996 Observation on bats in Gua Payau (Deer Cave), Gunung Mulu National Park, Sarawak. Sarawak Museum Journal 72:111-124.
A beach forest may comprise of large stands of beach forest vegetations and/or mangrove. Together with other beach vegetation like pokok kelapa or coconut trees (Cocos nucifera), Casuarina equisetifolia, with Dellinia suffruticosa and other palms, form a stable and strong barricade for huge waves that may otherwise erode the shoreline.


