Cucur atap Leaves
Baeckea frutescens L.
Myrtaceae
DEFINITION
Cucur atap leaves consist of the powder of dried leaves of Baeckea frutescens L. (Myrtaceae).
SYNONYM
Baeckea chinensis Gaertner, Baeckea cumingiana Schauer, Baeckea cochinchinensis Blume, Baeckea ericoides Schltdl, Baeckea stenophylla F.Muell, Baeckea sumatrana Blume [ 1 , 2 ].
VERNACULAR NAMES
False rue, shrubby baeckea (English); Cucur atap, chuchur atap, cucuran atap, hujong atap, rempah gunong, timor tasek, ujan atap (Malay); Gang son (Chinese) [ 1 , 2 ].
CHARACTER
| Colour | Greenish brown (powder) |
| Odour | Characteristic |
| Taste | Bitter |
IDENTIFICATION
Plant Morphology
B. frutescens is an evergreen, heather-like shrub or small tree up to 8 m tall. Bark greyish brown, fissured and flaky; branches upright, then spreading and drooping, with wiry ends. Leaves opposite, seemingly in clusters at condensed nodes, needle-like, 6–15 mm long x 0.4–0.8 mm wide, base narrowly cuneate, apex obtuse or acute, margin entire, resinous aromatic when crushed; petiole 0.5 mm long. Inflorescence axillary, 1-flowered; peduncle absent or very short; pedicel 0.8–1.7 mm long; bracteoles 2, early caducous. Flowers bisexual, 5-merous, hypanthium obconical to campanulate, 1.5–2.2 mm long, partly fused to the ovary; sepals semiorbicular, 0.4–0.9 mm long x 0.6–1.1 mm wide; corolla up to 5 mm across, petals orbicular, 1.1–1.8 mm across, white, oil glands present; stamens 7–13 in groups of 1–3 opposite each hypanthium lobe, filament 0.5–0.8 mm long, anther about 0.3 mm long; ovary 2–3 locular, 12–18 ovules per locule, style terete, about 1.2 mm long. Fruit a hemispherical to campanulate capsule opening by 3–4 longitudinal slits. Seedling with epigeal germination; cotyledons small, green; hypocotyl elongated [ 1 ].
Microscopy
Powdered material consists of abundant adaxial and abaxial epidermis cells with straight to wavy anticlinal wall, tetracytic stomata present only in abaxial epidermis. Brown coloured secretory cells found in group, sometimes associated with hypodermal tissue, brachysclereid cells, mesophyll palisade and parenchyma cells. The fragment of fibres found in groups or usually associated with vessels. Abundant of vessels are mostly of longitudinal pitted thickening and thick reticulated vessels. The very few starch granules are mostly simple, round to oval-shaped, usually found in group. Calcium oxalates consist of druses and prismatic crystals. Abundant brachysclereid cells are found in groups, very rarely isolated.

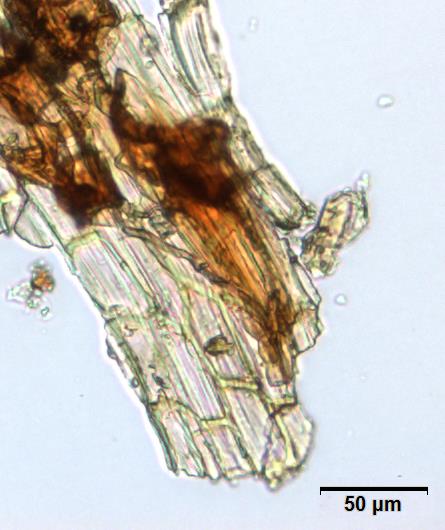


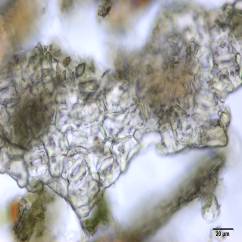

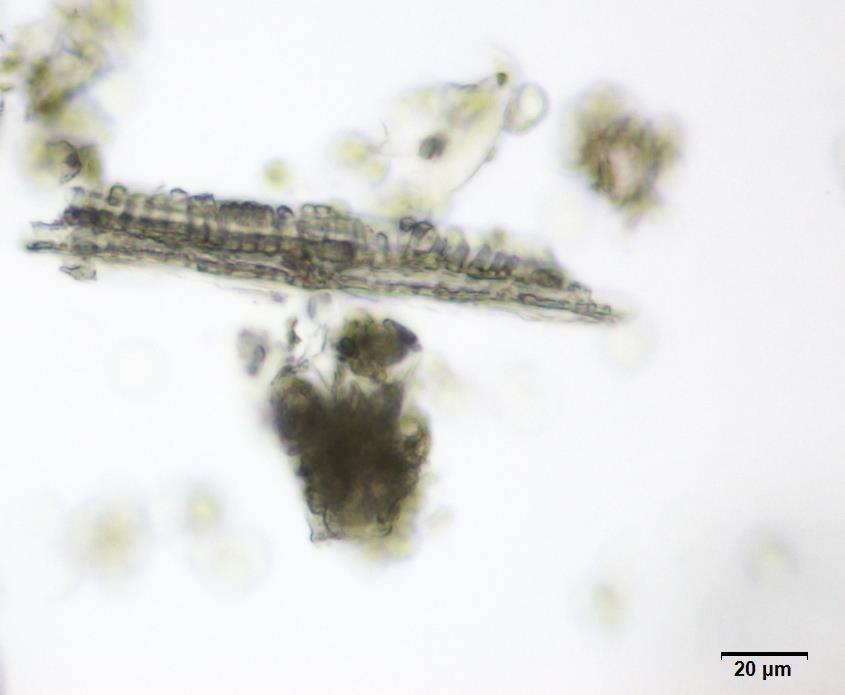

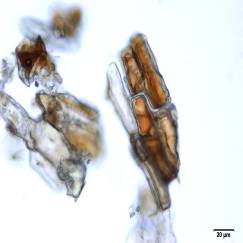
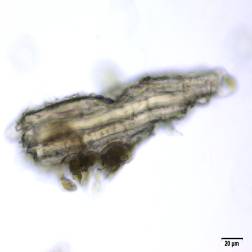
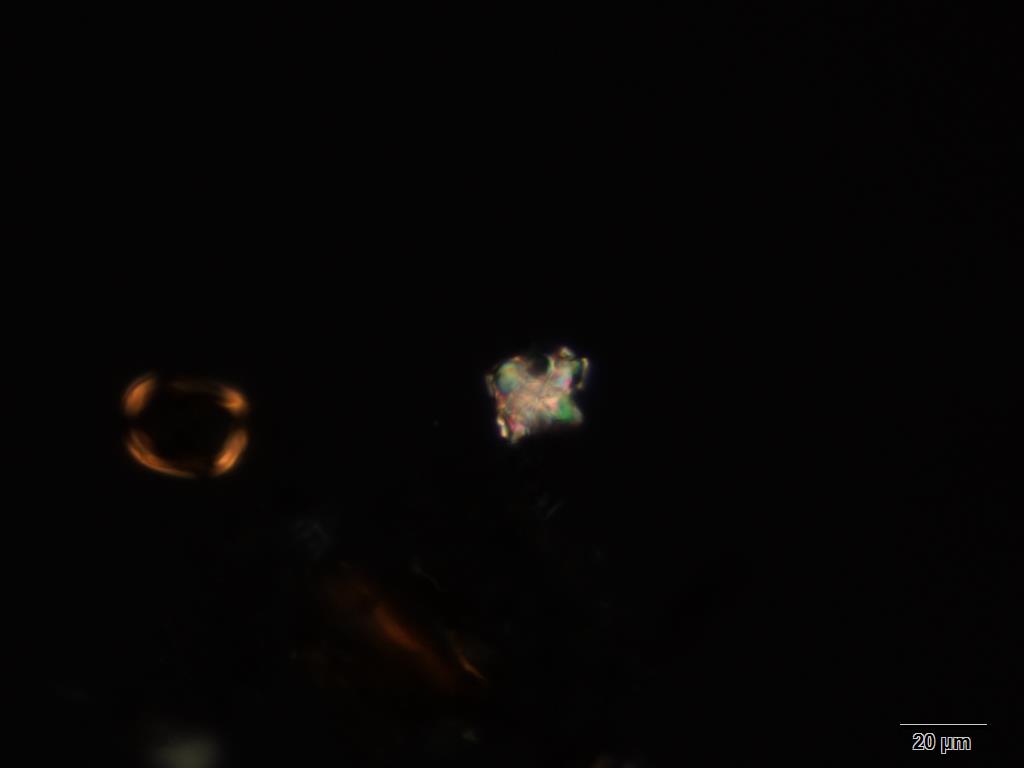
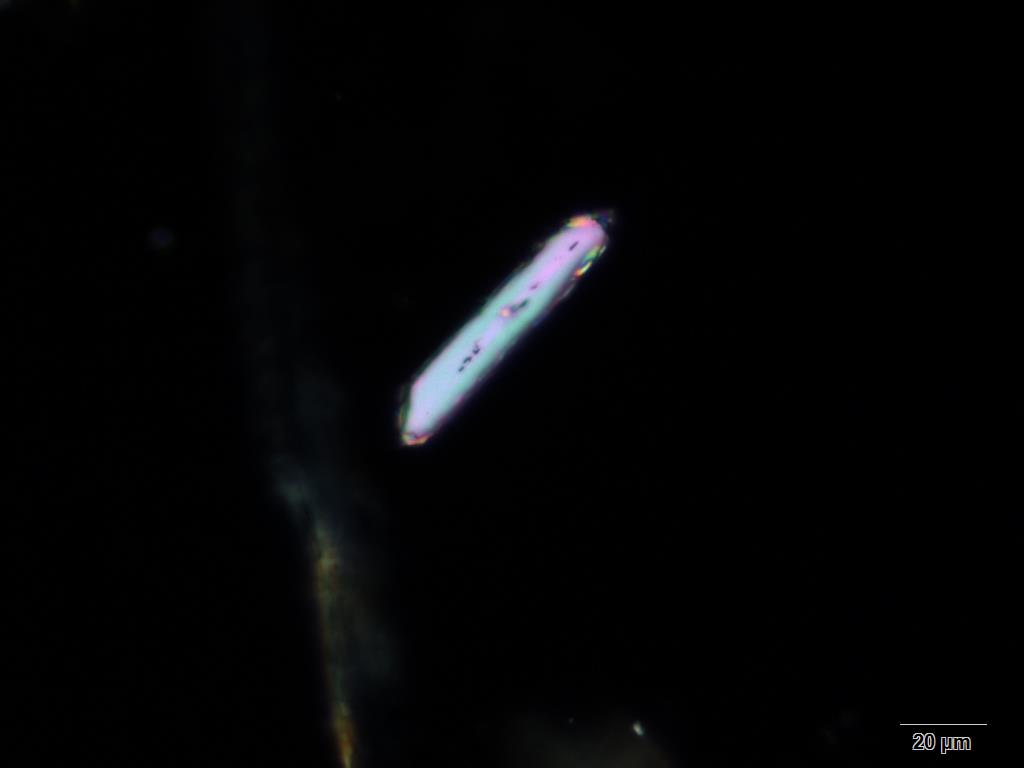
Figure 2 : Microscopic characters of Baeckea frutescens leaves powder of 0.355 mm size. (a) Epidermis cells (magnification 20x); (b) mesophyll palisade (magnification 10x); (c) starch granules (magnification 20x); (d) secretory cells (brown coloured) (magnification 20x); (e) adaxial epidermis cells attached with tetracytic stomata (arrow) (magnification 20x); (f) brachysclereid cells (arrow) (magnification 20x); (g) thick reticulated vessel (magnification 20x); (h) fragment of pitted vessel (magnification 20x); (i) mesophyll palisade (magnification 20x); (j) fragment of fibres (magnification 20x); (k) druse crystal [under polarizing filter] (magnification 10x); (l) prismatic crystal (magnification 20x). [Scale bars: a–l = 20 µm]
Colour Tests
Observed colour of solution after treatment with various reagents:
| HCl (conc.) | Yellow |
| NaOH (5%) | Brown |
Thin Layer Chromatography (TLC)
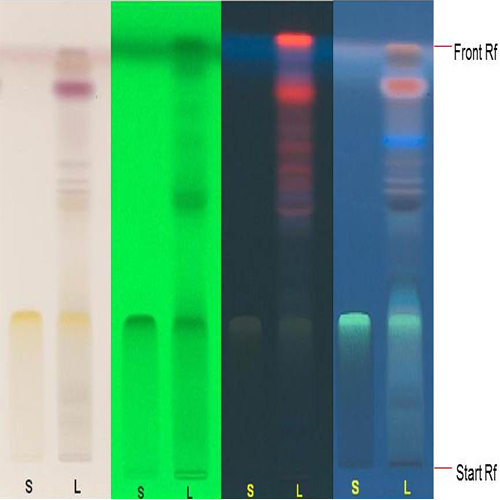
Figure 3 : TLC profiles of myricitrin standard (S) and ethanol extract of Baeckea frutescens dried leaves powder (L) observed under (a) visible light before derivatization, (b) UV at 254 nm before derivatization, (c) UV at 366 nm before derivatization and (d) UV at 366 nm after derivatization.
| Test Solutions | Weigh about 0.5 g of B. frutescens dried leaves powder in a screw cap conical flask and add 5 mL of ethanol. Sonicate for 15 minutes and filter the solution through filter paper. Evaporate the filtrate to dryness. Reconstitute with 5 mL methanol and use as a test solution. |
| Standard solution | Dissolve myricitrin standard [CAS no.: 17912-87-7] in methanol to produce 1.0 mg/mL solution. |
| Stationary Phase | HPTLC Glass Silica Gel 60 F254, 10 x 10 cm |
| Mobile phase | Chloroform : methanol : water; (6 : 4 : 1) (v/v/v), (with saturation pad) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
| Detection |
|
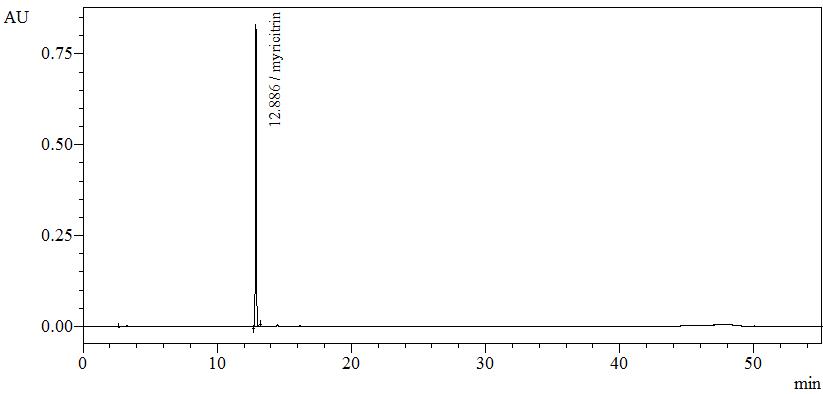
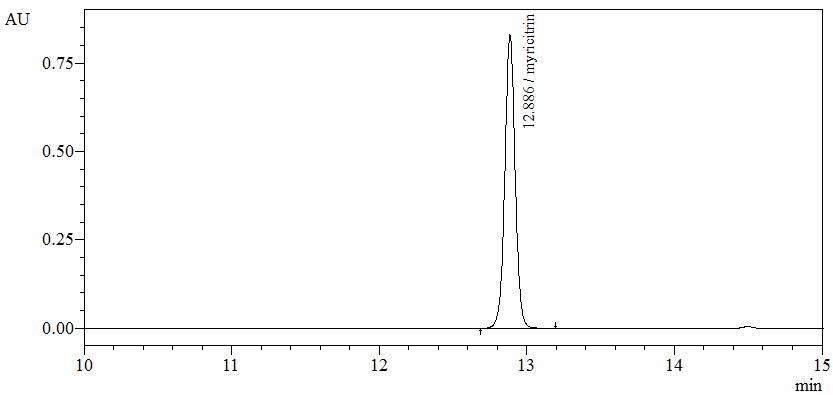
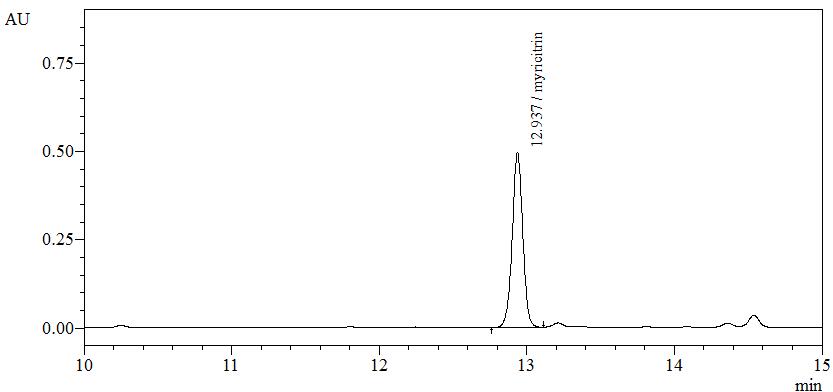
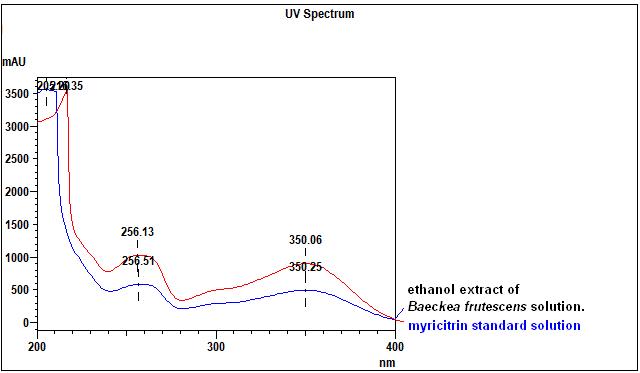
High Performance Liquid Chromatography (HPLC)
| Test solution | Weigh about 0.5 g of B. frutescens dried leaves powder in a screw cap conical flask and add 5 mL of ethanol. Sonicate for 15 minutes. Filter through a 0.45 µm syringe filter and inject the filtrate into the HPLC column. | ||||||||||||||||||
| Standard solution | Dissolve myricitrin standard [CAS no.: 17912-87-7] in methanol to produce 1.0 mg/mL solution. | ||||||||||||||||||
| Chromatographic system |
Detector: UV 350 nm Column: C18 column (5 µm, 4.6 mm I.D x 250 mm) (Zorbax Eclipse Plus C18 if necessary) Column oven temperature: 25°C Flow rate: 1.0 mL/min Injection volume: 2 µL |
||||||||||||||||||
| Mobile Phase (gradient mode) |
|
||||||||||||||||||
| System suitability requirement |
Perform at least five replicate injections of the standard solutions (1 mg/mL). The requirements of the system suitability parameters are as follow:
|
||||||||||||||||||
| Acceptance criteria |
|
PURITY TESTS
The purity tests are based on B. frustescens dried leaves powder of 0.355 mm particle size.
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 3% |
| Acid-insoluble ash | Not more than 1% |
| Loss on Drying |
| Not more than 10% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 13% |
| Cold method | Not less than 9% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 19% |
| Cold method | Not less than 6% |
SAFETY TESTS
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Methanol extract of B. frutescens leaves was found to contain chromones (e.g. 6β-C-glucopyranosyl-5,7-dihydroxy-2-methylchromone, 8β-C-glucopyranosyl-5,7-dihydroxy-2-methylchromone, 6β-C-(2′-galloylglucopyranosyl)-5,7-dihydroxy-2-methylchromone, 8β-C-(2′-galloylglucopyranosyl)-5,7-dihydroxy-2-methylchromone, 6β-C-glucopyranosyl-5,7-dihydroxy-2-isopropylchromone, 8β-C-glucopyranosyl-5,7-dihydroxy-2-isopropylchromone, 6β-C-(2′-galloylglucopyranosyl)-5,7-dihydroxy-2-isopropylchromone, 8β-C-(2′-galloylglucopyranosyl)-5,7-dihydroxy-2-isopropylchromone) and flavonoids (e.g. myricetin 3-O-α-L-rhamnopyranoside or myricitrin, 3-O-α-L-rhamnopyranosylmyricetinyl-(I-2″,II-2″)-3-O-α-L-rhamnopyranosylmyricetin) [ 3 , 4 ].
Ethanol extractof B. frutescens dried leaveswas found to contain phloroglucinol derivatives (baeckeol) and flavanones [ 5 , 6 ].
Dichloromethane extract of B. frutescens leaves was found to contain chromones (e.g. 5-hydroxy-7-methoxy-2-isopropylchromone, 5-hydroxyl-7-methoxy-2-isopropyl-8-methylchromone and 5 hydroxy-7-methoxy-2-iso-propyl-6-methylchromone) and chromanones (e.g. 2,5-dihydroxy-7-methoxy-2-isopropylchromanone, 2,5-dihydroxy-7-methoxy-2-isopropyl-8-methylchromanone, 2,5-dihydroxy-7-methoxy-2-isopropyl-6-methylchromanone, 2,5-dihydroxy-7-methoxy-2,8-dimethylchromanone and 2,5-dihydroxy-7-methoxy-2,6-dimethylchromanone) [ 7 ].
Essential oils of B. frutescens leaves was found to contain monoterpenes (e.g. 1,8-cineole, p-cymene, limonene, linalool, β-pinene, α-terpineol, terpinen-4-ol, γ-terpinene and α-thujene) and sesquiterpenes (e.g. β-caryophyllene, clovane-2,9-diol, α-eudesmol, β-eudesmol, γ-eudesmol and humulene epoxide) [ 8 , 9 , 10 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
Traditionally, the leaves of B. frutescens are made into a herbal tea and used to encourage menstrual flow and also for rubbing on the body during childbirth [ 1 , 11 ]. It is also traditionally used for fevers and as energy booster [ 11 ].
Biological and pharmacological activities supported by experimental data
Cytotoxic activity
Methanol extract from the dried leaves B. frutescens which produced phloroglucinol derivatives (BF-2), showed strong cytotoxic activity on leukemia cells (L1210) with inhibition concentration at 50% (IC50 = 5.0 µm/mL) in vitro [ 9 ].
Ethanol extract from the dried leaves B. frutescens which produced flavanones (BF-4 and BF-5), showed strong cytotoxic activity on leukemia cells (L1210) with inhibition concentration at 50% (IC50 = 0.2 and 0.5 µm/mL) in vitro [ 10 ].
Antibacterial activity
Ethanol extract of B. frutescens leaves (10 – 100 mg/mL) showed antibacterial effect to methicillin-resistant Staphylococcus aureus (MRSA), Staphylococcus aureus, Bacillus sp. and Escherichia coli with inhibition zones ranging from 7.0 to 14.5 mm compared to vancomycin using paper disc diffusion method [ 12 ].
Anticariogenic activity
Methanol extract (75%) of B. frutescens (1 – 20 mg/mL) leaves showed anticariogenic effect to Streptococcus mutans with microbial inhibition zones ranging from 9.0 to 14.0 mm using the well diffusion method [ 13 ].
Clinical studies
Information and data have not been established.
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Acute oral toxicity:
Oral single dose acute toxicity study on female Sprague Dawley rats (aged between 8 and 12 weeks old) using aqueous extract of B. frutescens leaves showed no toxicity effect on the parameters observed, including behaviors, body weight, food, and water intake. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. No-observed-adverse effect level(NOAEL) is more than 2,000 mg/kg body weight [ 14 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- Yusuf UK. Baeckea frutescens L.In: van Valkenburg JLCH, Bunyapraphatsara N. (Editors). Plant Resources of South-East Asia No. 12(2): Medicinal and poisonous plants 2. Backhuys Publisher, Leiden, The Netherlands. 2001. 96-98.
- Umberto Quattrocchi FLS. CRC World Dictionary of Medicinal and Poisonous Plants; common names scientific names, eponyms, synonyms and etymology. CRC Press. New York. 2012. pp. 517.
- Kamiya K, Satake T. Chemical constituents of Baeckea frutescens leaves inhibit copper-induced low-density lipoprotein oxidation. Fitoterapia. 2010;81:185-189.
- Tsui WY, Brown GD. Chromones and Chromanones from Baeckea frutescens. Phytochemistry. 1996;43(4):871-876.
- Fujimoto Y, Usui S, Makino M, Sumatra M. Phloroglucinolsfrom Baeckea frutescens. Phytochemistry 1996;41:923-925.
- Makino M, Fujimoto Y. Flavanonesfrom Baeckea frutescens. Phytochemistry. 1999;50:273-277.
- Satake T, Kamiya K, Saiki Y, Hama T, Fujimoto Y, Endang H. Chromone C-glycosides from Baeckea frutescens. Phytochemistry. 1999;50:303-306.
- Tsui WY, Brown GD. Sesquiterpenes from Baeckea frutescens. Journal of Natural Products. 1996;59:1084-1086.
- Ibrahim J, Abu Said A, Siti Aishah AB, Abdul Rashih A, Trockenbrodt M, Chak CV. Constituents of the essential oil of Baeckea frutescens L. from Malaysia. Flavour and Fragrance Journal. 1998;13:245-247.
- N’Guyen TT, Duong TT, Ange B, Vincent C, Alain M, Pascal R, Joseph C. Baeckea frutescens leaf oil from Vietnam: composition and chemical variability. Flavour and Fragrance Journal. 2004;19:217-220.
- Burkill IH. A Dictionary of the Economic Products of the Malay Peninsula. Vol. (1). Edited by: Ministry of Agriculture (Malaysia). Crown agents for the colonies, London. 1966. pp. 284-285.
- Somayeh R, Mahmood AA, Salmah I, Pouya H. Antibacterial activity of leaf extracts of Baeckea frutescens against Methicillin-Resistant Staphylococcus aureus. BioMed Research International. 2014:1-5.
- Hwang JK, Shim JS, Chung JY. Anticariogenic activity of some tropical medicinal plants against Streptococcus mutans. Fitoterapia. 2004;75:596-598.
- Norizah A, Nurulfariza R, Wan Mohammad Adham Afiq WZ, Teh BP, Hussin M. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of Health: 2015. Report no.: HMRC 11-045/01/BF/LT.