Roselle Calyx
Hibiscus sabdariffa L.
Malvaceae
DEFINITION
Whole or cut dried calyces and epicalyces of H. sabdariffa L. during fruiting.
SYNONYM
Abelmoschus cruentus (Bertol.) Walp., Furcaria sabdariffa Ulbr., Hibiscus cruentus Bertol., Hibiscus fraternus L., Hibiscus palmatilobus Baill., Sabdariffa rubra Kostel [ 1 ].
VERNACULAR NAMES
Asam susur (Malay), lou shen hua (Chinese), pulichai kerai (Tamil), rozelle, roselle or red sorrel (English) [ 2 , 3 ].
CHARACTER
The calyx powder is reddish colour with sour smell and acidic taste.
IDENTIFICATION
Plant Morphology
The calyx is joined in the lower half to form an urceolate structure, the upper half dividing to form 5 long acuminate recurved tips; the tips have a prominent, slightly protruding midrib and a large, thick nectar gland about 1 mm in diameter; the epicalyces consist of 8-12 small, obovate leaflets which are adnate to the base of the calyx; the calyces and epicalyces are fleshy, dry, easily fragmented and coloured bright-red to deep-purple, somewhat lighter at the base of the inner side [ 4 ].
Microscopy
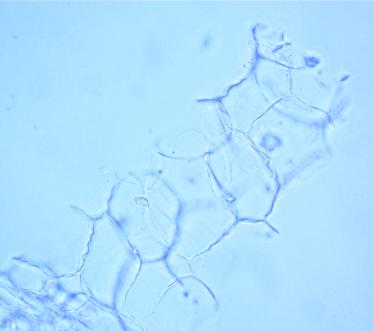
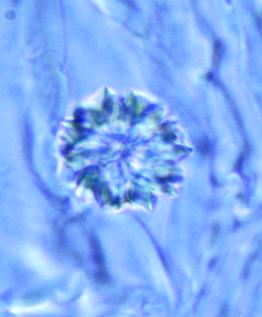
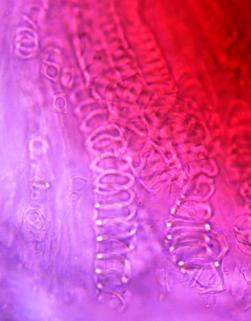
The powder shows predominantly red coloured fragments of the parenchyma containing numerous crystal clusters of calcium oxalate and, sporadically, mucilage filled cavities, sometimes associated with polygonal epidermal cells and anisocytic stomata; numerous fragments of vascular bundles with spiral and reticulate vessels; sclerenchymatous fibres with a wide lumen; rarely, rectangular, pitted parenchymatous cells; fragments of unicellular, smooth, bent covering trichomes and occasional glandular trichomes ; rounded pollen grains with spiny exine [ 4 ].
Colour Tests
Observed colour changes on treatment with various reagents:
| H2SO4 (conc.) | Brown |
| HCl (conc.) | Red |
| KOH (5%) | Green to dark green |
Thin Layer Chromatography (TLC)
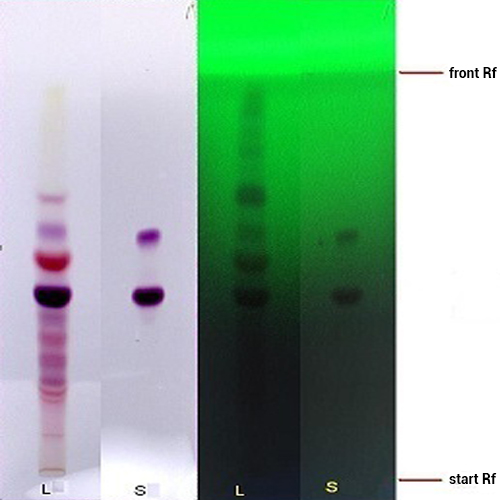
Figure 3 : TLC profiles of delphinidin-3-sambubioside standard (S) and ethanol extract of H. sabdariffa calyces (L) observed under (a) visible light and (b) UV at 254 nm.
| Test Solutions | Weigh about 5.0 g of of H. sabdariffa dried calyces powder in a 250 mL round bottom flask and add 80 mL ethanol into the flask. Reflux the sample for 1 hr and allow to cool. Filter the mixture and evaporate the solvent to dryness. Reconstitute the extract with 10 mL of methanol and use it as the test solution. |
| Standard solution | Dissolve 2.5 mg of delphinidin-3-sambubioside in 10 mL methanol to give 250 µg/mL solution. |
| Stationary Phase | HPTLC Silica gel 60 F254, 10 x 10 cm |
| Mobile phase | Anhydrous formic acid-water-butanol, 10:12:10 (v/v) |
| Application |
Pre-wash the HPTLC plate with chloroform-methanol (5:5, v/v) prior to application of the following solutions:
|
| Development distance | 8 cm (same direction with pre-wash) |
| Drying | Air drying |
| Detection |
|
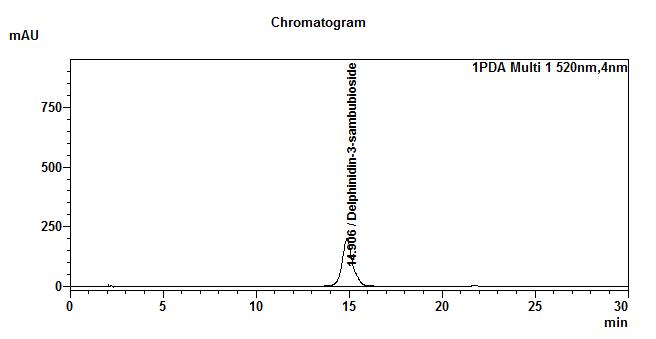
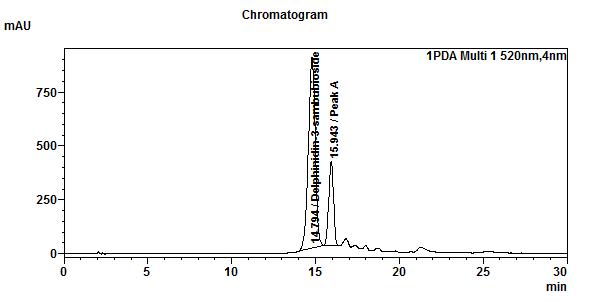
High Performance Liquid Chromatography (HPLC)
| Test solution | Extract about 1.0 g of H. sabdariffa dried calyces powder with 8 mL of methanol in a 50 mL screw-capped conical flask. Sonicate in a water bath of 40°C for 60 min. Cool the mixture and allow the insoluble matter to settle. Filter 3 mL of the upper solution through a 0.45 µm syringe filter and inject the filtrate into the HPLC column. | |||||||||||||||||||||
| Standard solution | Dissolve 2.5 mg of delphinidin-3-sambubioside standard with 10 mL of methanol to produce 250 µg/mL solution. | |||||||||||||||||||||
| Chromatographic system |
Detector: UV 520 nm Column: C18 (5 µm, 4.6 mm I.D x 150 mm) Column oven temperature: 30 °C Flow rate: 1.0 mL/min Injection volume: 20 µL |
|||||||||||||||||||||
| Mobile Phase (gradient mode) |
|
|||||||||||||||||||||
| System suitability requirement |
Perform at least five replicate injections of the delphinidin-3-sambubioside standard solution (250 µg/mL). The requirements of the system suitability parameters are as follow:
|
|||||||||||||||||||||
| Acceptance criteria |
|
PURITY TESTS
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 7% |
| Acid-insoluble ash | Not more than 1% |
| Loss on Drying |
| Not more than 10% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 52% |
| Cold method | Not less than 48% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 26% |
| Cold method | Not less than 21% |
SAFETY TEST
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
The calyces have been found to contain acids (e.g. citric acid, malic acid, tartaric acid, hibiscus acid, hydroxycitric acid, chlorogenic acid, chlorogenic acid isomers I and II, 5-O-caffeoylshikimic acid, protocatechuic acid), anthocyanins (e.g. delphinidin-3-O-sambubioside (hibiscin), delphinidin-3-monoglucoside, cyanidin-3-O-sambubioside, cyanidin-3-rutinoside), flavonoids (e.g. hibiscetin-3-monoglucoside (hibiscitrin), gossypetin-7-glucoside (gossypitrin), sabdaritrin, myricetin-3-arabinogalactose, quercetin-3-sambubioside, quercetin-3-rutinoside, quercetin-3-glucoside, kaempferol-3-O-rutinoside, kaempferol-3-(ρ-coumarylglucoside), quercetin) and others (e.g. 7-hydroxycoumarin, N-feruloytyramine, alkaloids, β-sitosterol, stearic acid, carotene, wax, mucilage, pectin, calcium citrate, hibiscin chloride) [ 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 ].
The fresh calyces had proteins, fats, fibres, minerals (e.g. iron, phosphorus, calcium, manganese, aluminium, magnesium, sodium, potassium) and vitamins (e.g. thiamine, riboflavin, niacin, ascorbic acid) [ 13 , 14 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
Traditionally used as a Thai traditional medicine for kidney stones. It is also used as an antibacterial, antifungal, hypocholesterolemic, diuretic, uricosuric and mild laxative [ 15 ].
Biological and pharmacological activities supported by experimental data
Protective effect of diabetic nephropathy Polyphenol from methanol extract of dried flower H. sabdariffa (up to 200 mg/kg) was given daily for 8 weeks via oral administration to streptozotocin-induced diabetic male Sprague Dawley rats which resulted in improved renal functions (high levels of creatinine and albumin with low level of blood urea nitrogen). Histological examination also showed lower hydropic change of renal proximal convoluted tubules in the rats. The levels of catalase and glutathione were increased whilst the levels of malondialdehyde and lipid peroxidation were lowered in the induced rats [ 16 ].
Antioxidant activity
Anthocyanins of ethanol extracts of dried flower H. sabdariffa were able to quench the free radicals of 1, 1-diphenyl-2-picrylhydrazyl (up to 0.50 mg/mL) with an IC50 value of 0.02 mg/mL. Whilst the lipid peroxidation which induced by tert-butylhydroperoxide (up to 0.20 mg/mL) on hepatocytes (pre-treated with the same extract) was significantly being inhibited (lowered concentration of malondialdehyde, p≤0.01) [ 17 ].
Hepatoprotective activity
Ethanol extract of dried flower of H. sabdariffa (up to 200 mg/kg) was given daily via oral administration to male Sprague Dawley rats and showed that the histological examination revealed no hepatotoxicity and hepatotoxicity reaction was significantly reduced (p< 0.05), in rats pre-treated with 100 and 200 mg/kg extracts after 5 days treatments [ 17 ].
Anti-inflammatory activity
Pre-incubated peripheral blood mononuclear cells with water extract of sun-dried calyces H. sabdariffa (up to 100 µg/mL) showed increment on levels of interleukin-6, interleukin-8, and monocyte chemoattractant protein-1 with the presence of H2O2 inflammatory stimuli, in a dose-dependent manner. Cell apoptosis was reduced [ 10 ].
Anticholesterol activity
The hot water extract of H. sabdariffa flower was given to males New Zealand White rabbits via oral administration daily (up to 1% w/v extract) for 10 weeks exhibited decreased the levels of serum cholesterol, triglycerides and low density lipoprotein-cholesterol [ 18 ].
Polyphenol from methanol extract of dried flower H. sabdariffa (up to 200 mg/kg) was given daily for 8 weeks continuously via oral administration to streptozotocin-induced diabetic male Sprague Dawley rats showed reduce serum levels of lipid such as total cholesterol, triglycerides and low density lipoprotein-cholesterol [ 16 ].
Clinical studies
In a human clinical trial, 10 healthy hospital staffs (5 adult per gender, aged 23-50 years) were ingested a single dose of 10 g of freeze-dried water extract of H. sabdariffa before measured levels of oxygen radical absorbance capacity (ORAC) in the plasma specimen and collected their venous bloods and plasma specimens at 0, 1.5, and 3 hour after consumption of the extract. Results showed some anti-oxidant activity in plasma. Whilst the levels of interleukin-6 and interleukin-8 remained low but level of monocyte chemoattractant protein-1 in each subject was decreased [ 10 ].
A study of 36 healthy male Thai medical students (aged 20-30 years) who consumed daily 16-24 g/day of H. sabdariffa juice for 7 days showed a decreased excretion of salts in urine such as creatinine, uric acid, citrate, tartrate, calcium, sodium, potassium and phosphate, except oxalate [ 19 ].
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Acute toxicity
Oral single dose acute toxicity study of female Sprague Dawley rats (aged between 8 and 12 weeks old) using aqueous mixture of H. sabdariffa dried calyces showed no toxic effect on the parameters observed which includes behaviors, body weight, food and water intakes. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. No-observed-adverse-effect level (NOAEL) is more than 2,000 mg/kg body weight[ 20 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Precaution
Caution use in individuals on diuretics, renally excreted medications, and/or narrow-therapeutic medications (such as digoxin, theophylline and phenytoin), as H. sabdariffa extracts have been reported to have a natriuretic effect [ 21 ].
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- The plant list. [Internet] Hibiscus sabdariffa; 2010 [cited on 7th December 2012]. Available from: http://www.theplantlist.org/tpl/record/kew-2850461
- Burkill IH. A dictionary of the economic products of the Malay Peninsula. Malaysia: Ministry of Agriculture. 1966;Vol 2:p. 1170-1171.
- Johannes S. World spice plants: economic usage, botany, taxonomy. European Union: Springer. 2005.
- British pharmacopoeia. Volume 1 & 2. London: The Stationery Office. 2007.
- Rao SP, Seshadri TR. Isolation of hibiscitrin from the flowers of Hibiscus sabdariffa: constitution of hibiscetin. Proceedings of the indian Academy of Science. 1942; 5A:148-153.
- Kerharo J. Senegal bisap (Hibiscus sabdariffa) or guinea sorrel or red sorrel. Planta Medica. 1971;5:277-281.
- Du CT, Francis FJ. Anthocyanins of rosella (Hibiscus sabdariffa, L.). Journal of Food Sciences. 1973;38:810-812.
- Khafaga E-SR, Koch H. Stage of maturity and quality of roselle (Hibiscus sabdariffa L. var. sabdariffa). 1. Organic acids. Angewandte. Botanik. 1980;54:287-293.
- Ojeda D, Jiménez-Ferrer E, Zamilpa A, Herrera-Arellano A, Tortoriello J, Alvarez L. Inhibition of angiotensin convertin enzyme (ACE) activity by the anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. Journal of Ethnopharmacology. 2010;127(1):7-10.
- Beltrán-Debón R, Alonso-Villaverde C, Aragonès G, Rodríguez-Medina I, Rull A, Micol V, Segura-Carretero A, Fernández-Gutiérrez A, Camps J, Joven J. The aqueous extract of Hibiscus sabdariffa calices modulates the production of monocyte chemoattractant protein-1 in humans. Phytomedicine. 2010;17(3-4):186-191.
- Fernández-Arroyo S, Rodríguez-Medina IC, Beltrán-Debón R, Pasini F, Joven J, Micol V, Segura-Carretero A, Fernández-Gutiérrez A. Quantification of the polyphenolic fraction and in vitro antioxidant and in vivo anti-hyperlipemic activities of Hibiscus sabdariffa aqueous extract. Food Research International. 2011;44(5):1490-1495.
- Vilasinee H, Anocha U, Noppawan PM, Nuntavan B, Hitoshi S, Angkana H, Chuthamanee S. Antioxidant effects of aqueous extracts from dried calyx of Hibiscus sabdariffa L. (roselle) in vitro using rat low-density lipoprotein (LDL). Biological & Pharmaceutical Bulletin. 2005;28(3):481-484.
- Guatam RD. Sorrel – a lesser-known source of medicinal soft drink and food in India. Natural Product Radiance. 2004;3(5);338-342.
- Mahadevan N, Shivali, Kamboj P. Hibiscus Sabdariffa Linn – an overview. Natural Product Radiance. 2009;8(1);77-83.
- Farnworth NR, Bunyapraphatsara N. Thai medicinal plants. Prachachon Press, Bangkok. 1992.p.163–166.
- Lee WC, Wang CJ, Chen YH, Hsu JD, Cheng SY, Chen HC, Lee HJ. Polyphenol extracts from Hibiscus sabdariffa Linnaeus attenuate nephropathy in experimental type 1 diabetes. Journal of Agricultural and Food Chemistry. 2009;57(6):2206-2210.
- Wang CJ, Wang JM, Lin WL, Chu CY, Chou FP, Tseng TH. Protective effect of Hibiscus anthocyanins against tert-butyl hydroperoxide-induced hepatic toxicity in rats. Food and Chemical Toxicology. 2000;38(5):411-416.
- Chen CC, Hsu JD, Wang SF, Chiang HC, Yang MY, Kao ES, Ho YC, Wang CJ. Hibiscus sabdariffa extract inhibits the development of atherosclerosis in cholesterol-fed rabbits. Journal of Agricultural and Food Chemistry. 2003;51(18):5472-5477.
- Kirdpon S, Nakorn SN, Kirdpon W.Changes in urinary chemical composition in healthy volunteers after consuming roselle (Hibiscus sabdariffa Linn.) juice. Journal of the Medical Association of Thailand. 1994;77:314–321.
- Teh BP, Hamzah NF, Rosli SNS, Yahaya MAF, Zakiah I, Murizal Z. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2012. Report No .: HMRC 11-045/01/HS/CLX2/A.
- Herrera-Arellano A, Flores-Romero S, Chavez-Soto MA, Tortoriello J. Effectiveness and tolerability of a standardized extract from Hibiscus sabdariffa in patients with mild to moderate hypertension: a controlled and randomized clinical trial. Phytomedicine. 2004;11(5):375-382.