Senduduk Leaves
Melastoma malabathricum L.
Melastomataceae
DEFINITION
Senduduk leaves consist of dried leaves of M. malabathricum (L.) (Melastomataceae).
SYNONYM
Melastoma affine D. Don., M. denticulatum Labill., M. polyanthum Blume.
VERNACULAR NAMES
Malabar melastone, Singapore rhododendron, straits rhododendron (English), senduduk, senduduk gunung, kuduk-kuduk (Malay), yě mōu dan (Chinese)
CHARACTER
The dried leaves powder has characteristic odour and tasteless.
IDENTIFICATION
Plant Morphology
Shrub or small tree 2-5 m tall. Stems reddish and covered with bristly scales; young branches quandrangular, covered with apprised, spreading or erect scales. Leaves elliptical to lanceolate, 1.5-5 cm x 2.5-9 cm, base rounded to acute, apex acuminate, 5- or 7-veined, above and beneath strigose to pilose; young leaves sour. Inflorencence cyme terminal, 3-12 flowered. Flowers purplish-pink, large (5-7cm) and showy, 5-10 growing at the end of a branch, normally 5-merous; hypanthium campanulate 5-11 mm x 1-10 mm, covered with long golden to red scales; sepals lanceolate, intersepalar emergences present; petals obovate, 15-35 mm x 10-22 mm, violet; stamens dimorphic; anther of longer outer stamens violet, connective prolonged, filaments 6-12 mm long; anther of inner stamens yellow, connective not prolonged, filaments 5.5-9.5 mm long. The flower opens only for one day at about 8 am and closing in the late afternoon, the petals falling off a few days later. The petals emerge from a cup-shaped calyx which remains after the flowers drop off. Shrubs with white flowers are sometimes seen. Fruits capsule, fleshy, berry-like 7-11 mm x 6-10 mm, opening irregularly transversely at maturity, exposing the soft dark blue pulp with orange seeds. Seeds stain the mouth when eaten, sweet and slightly astringent [ 1 , 2 , 3 , 4 , 5 , 6 ].
Microscopy
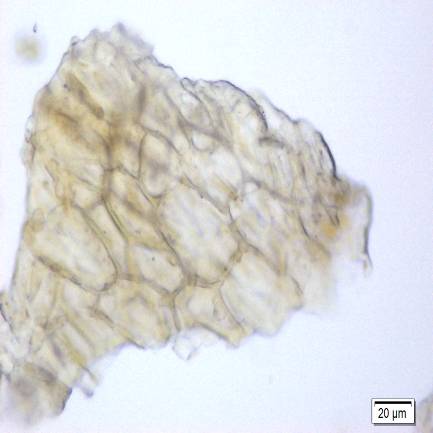



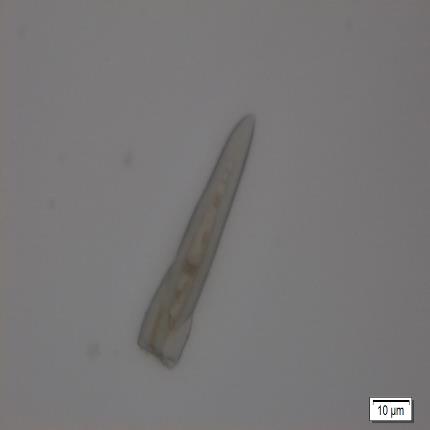

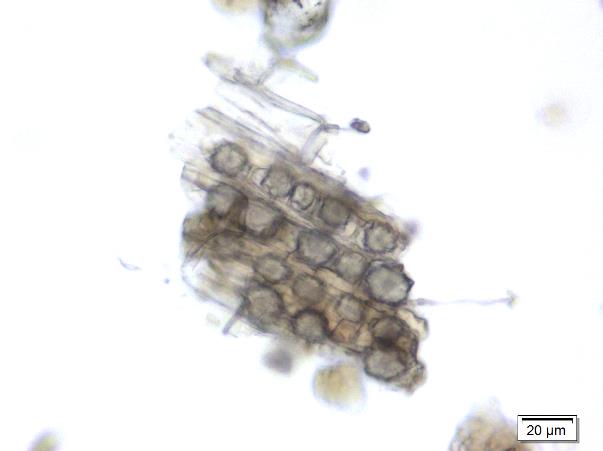
Figure 2 : Microscopic images of M. malabathricum leaf powder. (a) Epidermal cells (magnification 20x); (b) spirally thickened vessel (magnification 20x); (c) scalariform thickened vessel (magnification 20x); (d) simple trichome with the base (magnification 10x); (e) simple unicellular trichome (magnification 20x); (f) setose, multiseriate trichome (magnification 20x); (g) cluster of druse crystals (magnification 20x). [Scale bars: a-c, f, g = 20 µm; d=50 µm; e=10 µm]
Colour Tests
Observed colour of solution after treatment with various reagents:
| H2SO4 (conc.) | Brown |
| NaOH (5%) | Brown |
Thin Layer Chromatography (TLC)
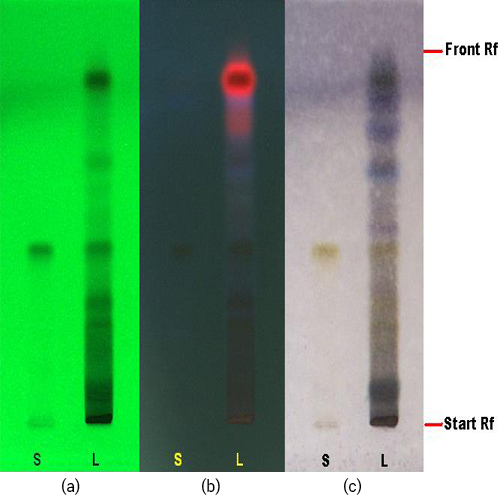
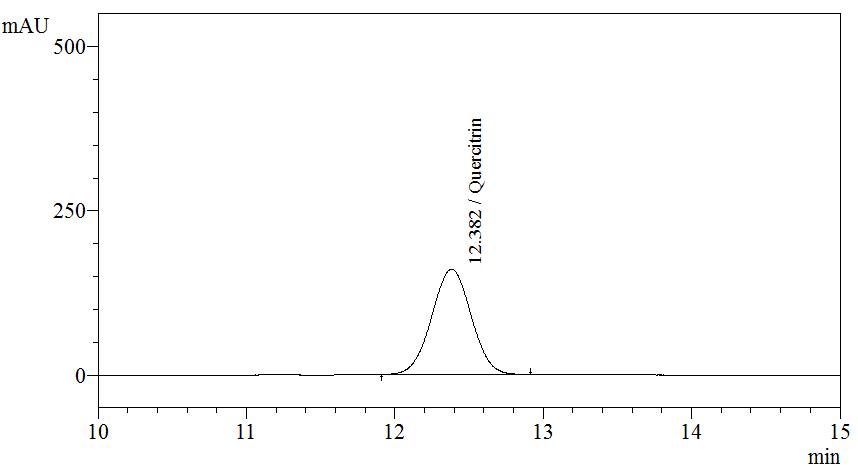
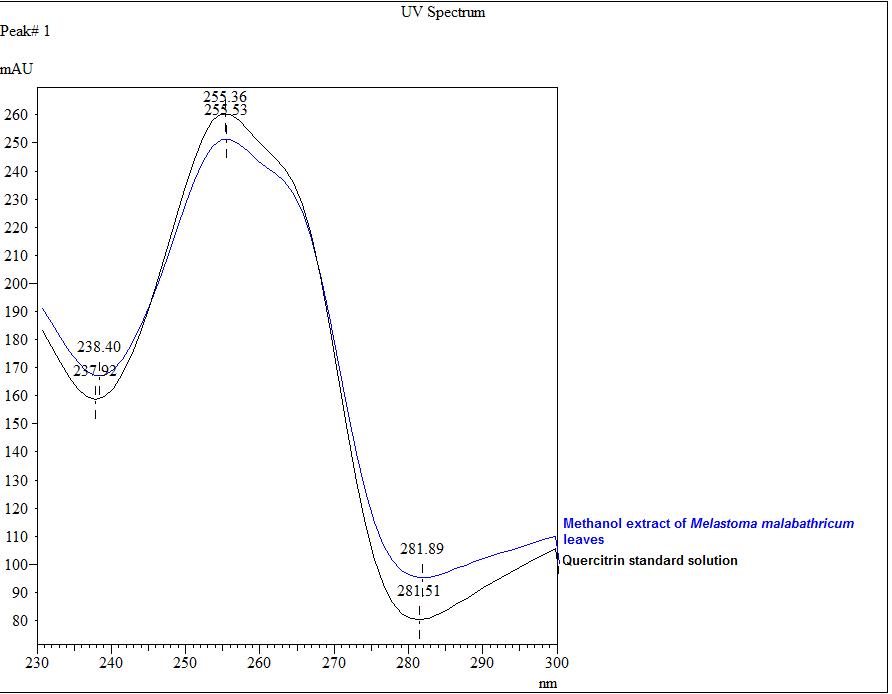
Figure 3 : TLC profiles of quercitrin (S) and methanol extract of M. malabathricum dried leaves methanol extract (L) observed under (a) UV at 254 nm before spray; (b) UV at 366 nm before spray; (c) visible light after spray.
| Test Solutions | Weigh about 2.0 g of M. malabathricum dried leaf powder in a 50 mL screw-capped conical flask and add 10 mL of methanol. Sonicate the mixture for 30 min and filter. Use the filtrate as test solution. |
| Standard solution | Dissolve 5.0 mg quercitrin standard in 10 mL methanol to produce 500 µg/mL. |
| Stationary Phase | HPTLC silica gel 60 F254, 5 x 10 cm |
| Mobile phase | Ethyl acetate : glacial acetic acid : formic acid : toluene; (20:3:3:5) (v/v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
| Detection |
|
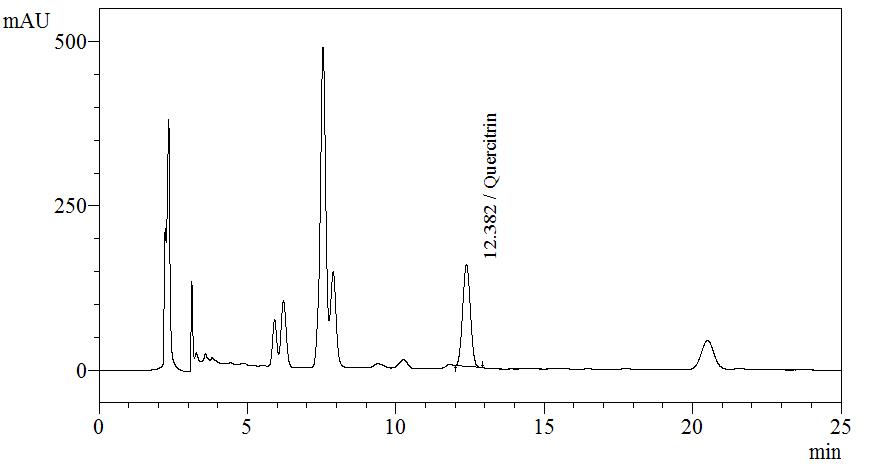
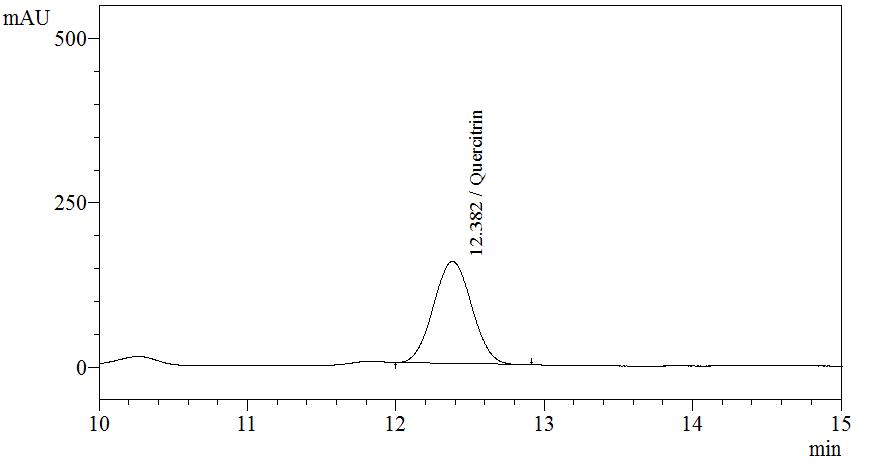
High Performance Liquid Chromatography (HPLC)
| Test solution | Extract about 1.0 g of M. malabathricum dried leaf powder in a 50 mL screw-capped conical flask with 10 mL of methanol. Sonicate the mixture for 30 min. Filter the solution through a 0.45 µm syringe filter and inject the filtrate into the HPLC column. |
| Standard solution | Dissolve 5.0 mg of quercitrin standard in 10 mL of methanol to produce 500 µg/mL solution |
| Chromatographic system |
Detector: UV 254 nm Column: C18 (5 µm, 4.6 mm I.D x 150 mm) Column oven temperature: 35°C Flow rate: 0.7 mL/min Injection volume: 10 µL |
| Mobile Phase (Isocratic mode) |
Isocratic elution using the mobile phase described below:
|
| System suitability requirement |
Perform at least five replicate injections of the standard solutions (500 µg/mL). The requirements of the system suitability parameters are as follow:
|
| Acceptance criteria |
|
PURITY TESTS
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 9% |
| Acid-insoluble ash | Not more than 1% |
| Loss on Drying |
| Not more than 10% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 14% |
| Cold method | Not less than 11% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 11% |
| Cold method | Not less than 10% |
SAFETY TESTS
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Aqueous methanolic extracts of the M. malabathricum leaves have been found to contain triterpenes and triterpenoids (e.g. β-sitosterol, β-sitosterol-3-O-β-D-glucopyranoside, ursolic acid, 2-hydroxyursolic acid and asiatic acid), flavonoids (e.g. quercetin and kaempferol) and others (e.g. glycerol-1,2-dilinolenyl-3-O-β-D-galactopyranoside and glycerol-1,2-dilinolenyl-3-O-(4,6-di-O-isopropylidene)-β-D-galactopyranoside) [ 7 , 8 , 9 , 10 ].
Methanol extract of the M. malabathricum leaves has been found to contain triterpenes and triterpenoids (e.g. β-sitosterol, β-sitosterol-3-O-β-D-glucopyranoside, ursolic acid, 2-hydroxyursolic acid, asiatic acid,α-amyrin and uvaol), flavonoids (e.g. rutin, quercetin and quercitrin) and others (e.g. glycerol-1,2-dilinolenyl-3-O-β-D-galactopyranoside and glycerol-1,2-dilinolenyl-3-O-(4,6-di-O-isopropylidene)-β-D-galactopyranoside) [ 7 , 8 ].
Ethanol extract of the M. malabathricum leaves has been found to contain volatile components, including (+)-3,4-dehydroproline amide, mefloquine, 2-(3,5-diphenyl-pyrazol-1-yl)-benzothiazole, 10′-bromo-9,9′ biphenanthryl-10-ol, 3,5-dichloro-2,4,6-trifluoropyridine, dehydro nylidrin acetate, N-(p-nitrophenyl)-P,P,P-triphenylphosphine imide, 2,6,10,14-tetramethylpentadecan-2-ol, 1,2,3,4-tetrahydro-4a,8a-ethenonaphthalene and 9-methyl-4,5-dihydro[1,2,5]oxadiazolo[3,4-f]cinnoline 3-oxide [ 11 ].
Acetone (70%) extract of the M. malabathricum leaves has been found to contain tannins (e.g. 1,4,6-tri-O-galloyl-β-D-glucoside, 1,2,4,6-tetra-O-galloyl-β-D-glucoside, malabathrins A-F, strictinin, casuarictin, casuarinin, pedunculagin, pterocarinin C, nobotanins B, D, G, H and J, (-)-epicatechin gallate, (-)-epicatechin, stachyurin, procyanidin B2 and B5, stenophyllanins A and B, and alienanin B) and others (e.g. brevifolincarboxylic acid and isoquercitrin 6”-O-gallate) [ 12 , 13 ].
MEDICINAL USES
Uses described in folk medicine not supported by experimental or clinical data
The decoction of M. malabathricum leaves and shoot-ends is used for diarrhea and decoction of a mixture of leaves and roots is used for women after childbirth. The decoction of M. malabathricum leaves, Zingiber officinale, Z. cassumunar and vinegar is also used for leucorrhea [ 14 ].
Traditionally, a handful of raw young shoots has been taken orally twice daily on an empty stomach for dysentery [ 17 ]. A powdered mixture of leaves and roots has been sprinkled over wound area to cure wound [ 14 ]. A paste of pounded leaves has also been applied topically to treat wound [ 16 ].
Biological and pharmacological activities supported by experimental data
Antimicrobial activity
Aqueous extract of M. malabathricum leaves (200 mg/mL) inhibited the growth of Bacillus brevis (inhibition zone of 21 mm), Vibrio cholera (20 mm), Candida kruesi (13 mm) and B. subtilis (13 mm) using agar cup method [ 17 ].
The extracts of M. malabathricum leaves (10 μg/mL) inhibited the radial growth of Colletotrichum gloeosporioides with the inhibition zone of 48.54 ± 0.41mm (acetone extract), 53.09 ± 0.75 mm (methanol extract) and 49.45 ± 0.32 mm (choloroform extract) using agar-disk dilution assay. In another test, these extracts inhibited the sporulation of C. gloeosporioides with the value of 2.24 ± 0.04 x 105 (acetone extract), 2.33 ± 0.04 x 105 (methanol extract) and 2.27 ± 0.04 x 105 (chloroform extract) spores using sporulation assay. The MIC value of all the extracts was determined at 20 μg/mL [ 18 ].
Antihelmintic activity
Haemonchus contortus larval development was significantly (p < 0.05) inhibited by M. malabathricum leaf extracts at a concentration of 50 mg/mL using larva development assay. The inhibition was 77.00% (aqueous extract), 79.48% (90% of ethanol extract) and 61.60% (chloroform extract) [ 19 ].
H. contortus egg hatching was significantly (p < 0.05) inhibited by M. malabathricum leaf extracts at a concentration of 50 mg/mL using egg hatch assay. The inhibition was 84.44% (aqueous extract), 86.05% (90% of ethanol extract) and 49.39% (chloroform extract) [ 19 ].
The motility of adult H. contortus was completely inhibited by aqueous extract of M. malabathricum leaves (50 mg/mL) after eight hours exposure using adult motility assay [ 19 ].
Anti-ulcer activity
Methanol extract of M. malabathricum leaves (50-500 mg/kg/day) administered orally to adult male Sprague Dawley rats seven days before the induction of gastric ulcer using ethanol significantly (p < 0.05) decreased ulcer formation [ 20 ].
Aqueous extract of M. malabathricum leaves (250 and 500 mg/kg) administered orally as a single dose to adult male Sprague Dawley rats one hour before the induction of gastric ulcer using ethanol significantly (p < 0.05) decreased ulcer formation and showed lack of submucosal edema and no leucocytes infiltration [ 21 ].
Ulcerogenic activity
Methanol extract of M. malabathricum leaves (50-500 mg/kg/day) administered orally to adult male Sprague Dawley rats seven days before the induction of gastric ulcer using indomethacin significantly (p <0.05) increased ulcer formation [ 22 ].
Antidiarrheal activity
Aqueous extract of M. malabathricum leaves (100-500 mg/kg) administered orally as a single dose to Swiss mice. After 12 hours, a significant (p < 0.05) decrease in fecal output was observed in treated mice [ 22 ].
Aqueous extract of M. malabathricum leaves (100-500 mg/kg) administered orally as a single dose to Swiss mice one hour before the induction of diarrhea using castor oil increased the percentage of protection (60-80%) against diarrhoea [ 22 ].
Aqueous extract of M. malabathricum leaves (100-500 mg/kg) administered orally as a single dose to Swiss mice one hour before the induction of diarrhea using magnesium sulphate significantly (p < 0.05) decreased the intestinal fluid secretion [ 22 ].
Aqueous extract of M. malabathricum leaves (100-500 mg/kg) administered orally as a single dose to Swiss mice before the administration of charcoal marker. After 30 minutes, a significant (p < 0.05) inhibition of small intestine motility of the charcoal marker was observed in treated mice [ 22 ].
Anti-inflammatory activity
Ethanol extract of M. malabathricum leaves (250 and 500 mg/kg) were administered orally as single doses to adult male and female Wistar albino rats before the induction of paw edema using carrageenan. After three hours, a significant (p < 0.05 for 250 mg/kg, p < 0.001 for 500 mg/kg) inhibition of paw edema was observed in treated rats [ 23 ].
Aqueous extract of M. malabathricum leaves (24.35-48.70 mg/kg) administered subcutaneously as single dose to Sprague Dawley rats (aged 8-10 weeks) 30 minutes before induction of paw edema with carrageenan significantly (p < 0.05) inhibited paw edema [ 24 ].
Anticoagulant activity
Activated partial thromboplastin time (APTT) was significantly (p < 0.05) prolonged by hot water (180 s), cold water (120 ± 0.9 s) and methanol (108 ± 0.7 s) extracts of M. malabathricum leaves (1mg/mL) compared to deionized water (38.9 ± 0.5 s). Prothrombin time (PT) was significantly (p < 0.05) prolonged by only hot (20.0 ± 1.3 s) and cold water (16.4 ± 0.4) extracts compared to control (13.3 ± 0.5 s) [ 25 ].
Hot aqueous extract of M. malabathricum leaves (100-800 µg/mL) showed a significant (p < 0.001) prolongation of the APTT in a concentration dependent manner with heparin as positive control using clot-based assay [ 25 ].
Anticancer activity
Methanol extract of M. malabathricum leaves (12.5-100 µg/mL) showed antiproliferative activity on estrogen-dependent human breast adenocarcinoma (MCF-7), human cervical adenocarcinoma (HeLa), human ovarian adenocarcinoma (Caov-3), acute promyelocytic leukemia (HL-60), T-lymphoblastic leukemia (CEM-SS) and human breast cancer (MDA-MB-231) cells (IC50 values of MCF-7 = 87 µg/mL, HeLa = 88 µg/ml, Caov-3 = 41 µg/mL, HL-60 = 13 µg/mL, CEM-SS = 30 µg/mL and MDA-MB-231 = 59 µg/mL) [ 26 ].
Anti-oxidant activity
The total phenolic content of M. malabathricum leaves (6.25 mg/mL each) are 3055 mg/100 g gallic acid (methanol extract) and 3344 mg/100 g gallic acid (aqueous extract). The extracts (500 µg/ml) showed high anti-oxidant activity with DPPH radical scavenging activity of 97.3% (methanol extract) and 69.8% (aqueous extract). The extracts (20 – 500 µg/ml) showed high superoxide scavenging activity of 91%-99% (methanol extract) and 80%-96% (aqueous extract) [ 26 ].
The extracts of M. malabathricum leaves (200 µg/mL each) showed high anti-oxidant activity 81.74% (methanol extract) and 83.28% (chloroform extract) compared to quercetin (68.54%) using β-carotene bleaching assay [ 10 ].
Antinociceptive activity
Aqueous extract of M. malabathricum leaves (4.87-48.7 mg/kg) administered subcutaneously as a single dose to male BALB/c mice (aged 5-7 weeks) 30 minutes before the induction of abdominal constriction using acetic acid significantly (p < 0.05) showed antinociceptive activity by decreasing the number of abdominal constrictions in the treated mice [ 24 ].
Aqueous extract of M. malabathricum leaves (4.87-48.7 mg/kg) administered subcutaneously as a single dose to male BALB/c mice (aged 5-7 weeks) 30 minutes before the hot plate-test was conducted. A significant (p < 0.05) prolongation of the latency time was observed in treated mice [ 24 ].
Aqueous extract of M. malabathricum leaves (4.87-48.7 mg/kg) administered subcutaneously as a single dose to Sprague Dawley rats (aged 8-10 weeks) 30 minutes before the induction of pain using formalin significantly (p < 0.05) showed antinociceptive activity compared to water [ 24 ].
Antipyretic activity
Aqueous extract of M. malabathricum leaves (4.87-48.7 mg/kg) administered subcutaneously as a single dose to Sprague Dawley rats (aged 8-10 weeks) 30 minutes before the induction of pyrexia using Brewer’s yeast significantly (p < 0.05) showed antipyretic activity compared to water [ 24 ].
Hepatoprotective activity
Methanol extract of M. malabathricum leaves (250-500 mg/kg/day) administered orally to adult male Sprague Dawley rats seven days before the induction of liver toxicity using either paracetamol or carbon tetrachloride significantly (p < 0.05) reduced the level of liver enzymes aspartate transaminase and alanine transaminase, the severity of hepatocytes necrosis and hemorrhage compared to control [ 27 ].
Reproductive activity
Ethanol extract of M. malabathricum leaves (500 mg/kg/day) administered orally to male Wistar albino rats for 14 days significantly (p < 0.01) increased the weight of male reproductive organs (testis, epididymis, seminal vesicle, ventral prostrate and vas deferens), sperm density and sperm motility. The extract also increased serum antioxidants which are catalase (p < 0.01), glutathione peroxidase (p < 0.05), glutathione-S transferase (p < 0.05), superoxide dismutase (p < 0.01) and glutathione reductase (p < 0.01), the activity of liver marker enzymes (serum glutamate pyruvate transaminase, serum glutamate oxaloacetate transaminase and serum alkaline phosphatase), the reproductive hormone level (testosterone (p < 0.05) and luteinizing hormone (p < 0.05)) and decreased estrogen (p < 0.05) in treated rats [ 29 ].
Ethanol extract of M. malabathricum leaves (500 mg/kg/day) was administered orally to male Wistar albino rats 14 days before being placed in an individual cage with two untreated females of the same strains. The study was conducted for duration of 10 days which showed the increased number of pregnant females, implantation, viable fetus and resorption sites [ 29 ].
Clinical studies
Information and data have not been established.
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Acute toxicity
Aqueous extract of M. malabathricum leaves (62.5-2000 mg/kg) was administered orally as a single dose to Swiss mice. The study was conducted for duration of 48 hours and showed no toxic effect (LD50 value > 2000 mg/kg) [ 22 ].
Ethanol extract of M. malabathricum leaves (200-2000 mg/kg) was administered orally as a single dose to adult male and female Wistar albino rats. The study was conducted for duration of 72 hours and showed no toxic effect (LD50 value > 2000 mg/kg) [ 23 ].
Methanol extract of M. malabathricum leaves (5000 mg/kg/day) was administered orally as a single dose to adult male Sprague Dawley rats. The study was conducted for 14 days and showed no toxic effect (LD50 value > 5000 mg/kg) [ 27 ].
Oral single dose acute toxicity study on female Sprague Dawley rats (aged between 8 to 12 weeks old) using aqueous mixture of powdered M. malabathricum leaves showed no toxic effect on the parameters observed which includes behavior, body weight, food and water intakes. All rats were observed for 14 days and no death was found throughout the study period. No-observed-adverse-effect level (NOAEL) is more than 2,000 mg/kg body weight [ 28 ].
Ethanol extract of M. malabathricum leaves (2000 mg/kg/day) was administered orally as a single dose to adult male Sprague Dawley rats. The study was conducted for 14 days and showed no toxic effect with LD50 value > 2000 mg/kg [ 29 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- Valkenburg JLCH, Bunyapraphatsara N. Medicinal and poisonous plants 2. PROSEA;p.365-366.
- Foo TS. Guide to the wildflowers of Singapore. Singapore Science Centre; 1985;p.160.
- Wee YC. A guide to medicinal plants. The Singapore Science Centre; p.160.
- Hsuan K, Chin SC, Tan HTW. The concise flora of Singapore: gymnosperms and dicotyledons. Singapore University Press;p.222.
- Corners EJH. Wayside trees of Malaya Volume 1. Fourth edition, Malayan Nature Society, Kuala Lumpur. 1997;p.1-476, plates 1-38.
- Burkill IH. A dictionary of the economic products of the Malay Peninsula. Volume 1. 3rd printing. Publication Unit, Ministry of Agriculture, Malaysia, Kuala Lumpur. 1993;p.1-1240.
- Nuresti S, Baek SH, Asari A. Chemical components of Melastoma malabathricum. ACGC Chemical Research Communications. 2003;16:28-33.
- Ali DMH, Wong KC, Boey PL. Triterpenoids, glycolipids and flavonoids of Melastoma malabathricum: Isolation, spectrometric characterization and antibacterial activity. Germany: VDM Verlag Dr. Muller Aktiengesellschaft & Co. KG. 2010.
- Wong KC, Ali DMH, Boey PL. Chemical constituents and antibacterial activity of Melastoma malabathricum L. Natural Products Research. 2012;26(7):609-618.
- Karupiah S, Ismail Z. Antioxidative effect of Melastoma malabathricum L. extract and determination of its bioactive flavonoids from various locations in Malaysia by RP-HPLC with diode array detection. Journal of Applied Pharmaceutical Science. 2013;3(2):19-24.
- Balamurugan K, Nishanthini A, Lalitharani S, Mohan VR. GC-MS determination of bioactive components of Melastoma malabathricum L. International Journal of Current Pharmaceutical Research. 2012;4(4):24-26.
- Yoshida T, Nakata F, Hosotani K, Nitta A, Okuda T. Dimeric hydrolysable tannins from Melastoma malabathricum. Phytochemistry. 1992;31:2829-2833.
- Yoshida T, Nakata F, Hosotani K, Nitta A, Okuda T. Tannins and related polyphenols of melastomataceous plants. V. Three new complex tannins from Melastoma malabathricum L. Chemical & Pharmaceutical Bulletin. 1992;40(7):1727-1732.
- Burkill IH. A dictionary of the economic products of the Malay peninsula. Kuala Lumpur: Ministry of Agriculture Malaysia, 1935;p.1440.
- Sajem AL, Gosai K. Traditional use of medicinal plants by the Jaintia tribes in North Cachar Hills district of Assam, northeast India. Journal of Ethnobiology and Ethnomedicine. 2006;2:33.
- Ahmad FB, Ismail G. Medicinal plants used by Kadazandusun communities around Crocker range. ASEAN Review of Biodiversity and Environmental Conservation. 2003;p.1-10.
- Thatoi HN, Panda SK, Rath SK, Dutta SK. Antimicrobial activity and ethnomedicinal uses of some medicinal plants from Similipal Biosphere Reserve, Orissa. Asian Journal of Plant Sciences. 2008;7(3):260-267.
- Johnny L, Yusuf UK, Nulit R. The effect of herbal plant extracts on the growth and sporulation of Colletotrichum gloeosporioides. Journal of Applied Biosciences. 2010;34:2218-2224.
- Suteky T, Dwatmadji. Antihelmintic activity of Melastoma malabatricum extract on Haemonchus contortus activity in vitro. Asian Journal of Pharmaceutical and Clinical Research. 2011;4(Suppl 1):68-70.
- Zabidi Z, Wan Zainulddin WN, Mamat SS, Shamsahal Din S, Kamisan FH, Yahya F, Ismail NA, Rodzi R, Hassan H, Mohtarrudin N, Somchit MN, Zakaria ZA. Antiulcer activity of methanol extract of Melastoma malabathricum leaves in rats. Medical Principles and Practice. 2012; 21:501–503.
- Hussain F, Abdulla MA, Mohd Noor S, Ismail S, Mohd Ali H. Gastroprotective effects of Melastoma malabathricum aqueous leaf extract against ethanol-induced gastric ulcer in rats. American Journal of Biochemistry and Biotechnology. 2008;4(4):438-441.
- Sunilson JAJ, Anandarajagopal K, Kumari AVAG, Mohan S. Antidiarrhoeal activity of leaves of Melastoma malabathricum Linn. Indian Journal of Pharmaceutical Sciences. 2009;71(6):691–695.
- Balamurugan K, Sakthidevi G, Mohan VR. Anti-inflammatory activity of leaf of Melastoma malabathricum L. (Melastomaceae). International Journal of Research in Ayurveda and Pharmacy. 2012;3(6):801-802.
- Zakaria ZA, Raden Mohd. Nor RNS, Hanan Kumar G, Abdul Ghani ZDF, Sulaiman MR, Rathna Devi G, Mat Jais AM, Somchit MN, Fatimah CA. Antinociceptive, anti-inflammatory and antipyretic properties of Melastoma malabathricum leaves aqueous extract in experimental animals. Canadian Journal of Physiology and Pharmacology. 2006;84:1291–1299
- Manicam C, Abdullah JO, Mohd Tohit ER, Seman Z, Chin SC, Hamid M. In vitro anticoagulant activities of Melastoma malabathricum Linn. aqueous leaf extract: a preliminary novel finding. Journal of Medicinal Plants Research. 2010;4(14):1464-1472.
- Zakaria ZA, Rofiee MS, Mohamed AM, Teh LK, Salleh MZ. In vitro antiproliferative and antioxidant activities and total phenolic contents of the extracts of Melastoma malabathricum leaves. Journal of Acupuncture and Meridian Studies. 2011;4(4):248-256.
- KamisanFH, Yahya F, IsmailNA, Shamsahal DinS, MamatSS, Zabidi Z, Wan Zainulddin WN, MohtarrudinN, HusainH, AhmadZ, Zakaria ZA. Hepatoprotective activity of methanol extract of Melastoma malabathricum leaf in rats. Journal of Acupuncture and Meridian Studies. 2013;61: 52-55.
- Teh BP, Hamzah NF, Rosli SNS, Yahaya MAF, Zakiah I, Murizal Z. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2012. Report No.: HMRC 11-045/01/MM/L/J.
- Balamurugan K, Sakthidevi G, Mohan VR. Stimulatory effect of the ethanol extract of Melastoma malabathricum L. (Melastomataceae) leaf on the reproductive system of male albino rats. Journal of Applied Pharmaceutical Science. 2013;3(02):160-165.