Dukung Anak Herb
Phyllanthus niruri L.
Phyllanthaceae
DEFINITION
Dukung anak herb consists of the dried aerial part of P. niruri L.
SYNONYM
None.
VERNACULAR NAMES
Dukung anak, dukong-dukong anak, amin buah, rami buah, turi hutan (Malay); zhen chu cao, ye xia zhu (Chinese); keelaanelli (Tamil); child pick-a-back (English) [ 1 , 2 ].
CHARACTER
Dukung anak is light green in colour with slight odour and bitter taste.
IDENTIFICATION
Plant Morphology
P. niruri is a small erect annual herb growing up to 30-60 cm in height with a slim, round or angular, smooth and light green stem; the branches are spreading and close-set with pinnate compound leaves. The leaflets are green, entire and alternately arranged with an elliptic-oblong or elliptical blade, 5-20 mm long and 2-3 mm wide. The flowers are found at the underside of leaf rachis, very numerous, small, yellow, pale green or greenish white, which are often flushed in red; monoecious with 1-3 male/staminate flowers, and solitary female/pistillate flowers borne axillary and 6 sepals. Fruits capsule, very small, green, globose, smooth containing seeds and on the underside of leaf rachis. Seeds pale brown with 6-7 straight longitudinal ribs on the back [ 3 , 4 , 5 ].
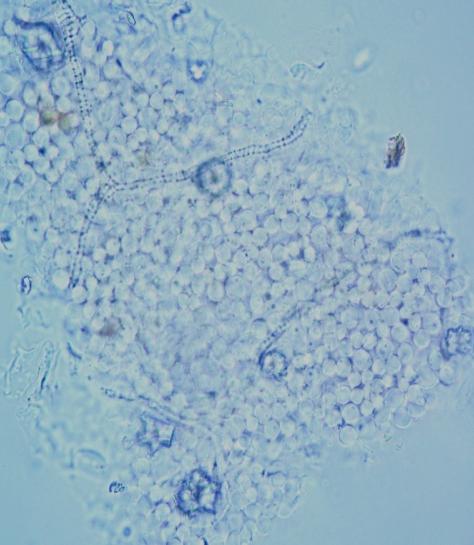
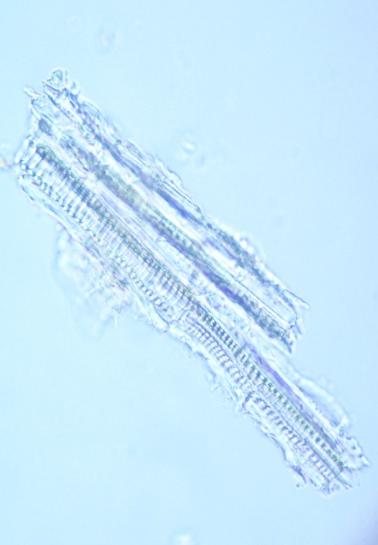
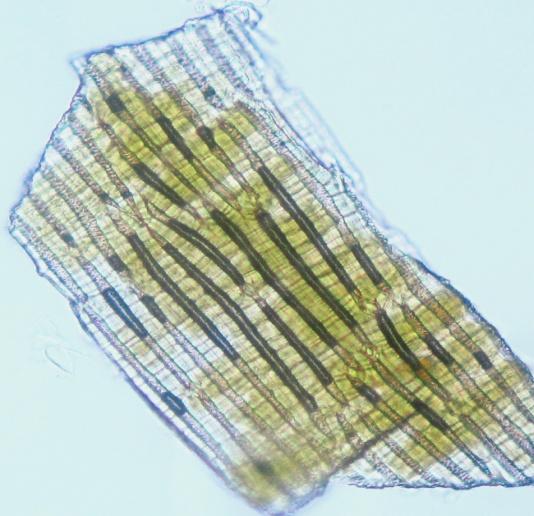
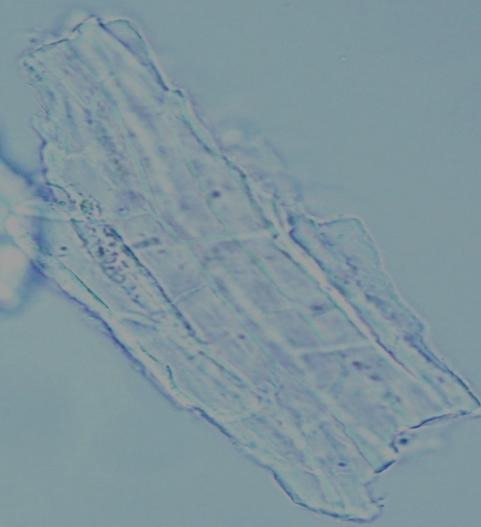
Microscopy
The powder consists of fragment of leaf epidermis that composed of cells with sinuous walls and anisocytic stomata, palisade cells, parenchyma cells prismatic and rosette-shaped calcium oxalate crystals, spiral vessels and fragments of epicarp and seeds coat.
Colour Tests
Observed colour of solution after treatment with various reagents:
| HCl (conc) | Green |
| NaOH (5%) | Brown |
| KOH (5%) | Brown |
| FeCI3 | Green to dark green |
Thin Layer Chromatography (TLC)
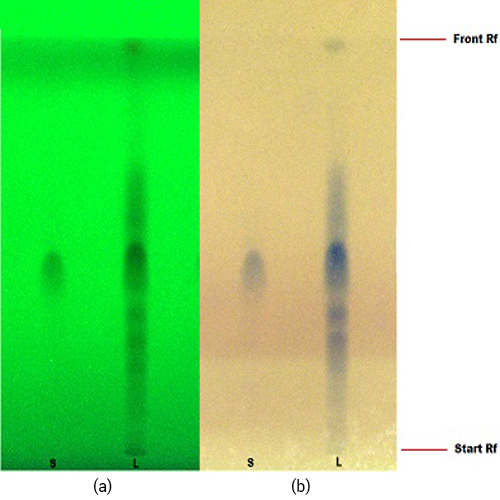
Figure 3 : TLC profiles of corilagin (S) and methanol extract of P. niruri herb (L) observed under (a) UV at 254 nm (b) visible light spray
| Test Solutions | Weigh about 0.5 g of of P. niruri dried herb powder in a 50 mL screw-capped conical flask and add 10 mL of methanol into the flask. Sonicate the mixture for 15 min. Filter and use the filtrate as test solution. |
| Standard solution | Dissolve 5.0 mg corilagin standard in 10 mL methanol to give 500 µg/mL. |
| Stationary Phase | HPTLC Silica gel 60 F254, 5 x 10 cm |
| Mobile phase | Ethyl acetate-glacial acetic acid-formic acid-water, 25:3:3:5 (v/v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
| Detection |
|
High Performance Liquid Chromatography (HPLC)
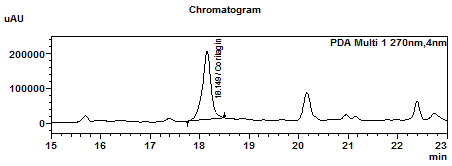
| Test solution | Extract about 2.0 g of P. niruri dried herb powder in a 50 mL screw-capped conical flask and add 20 mL of ethanol. Shake the mixture for 15 min and filter. Evaporate the filtrate to dryness over a water bath. Reconstitute the dried extract with 0.5 mL of methanol. Filter the solution through a 0.45 µm syringe filter and inject the filtrate into the HPLC column. | |||||||||||||||
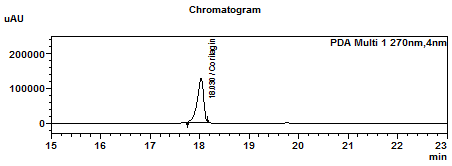
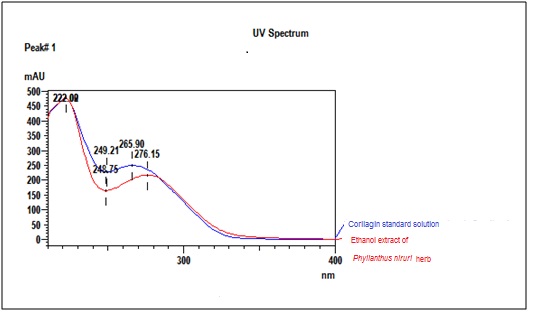
| Standard solution | Dissolve 2.0 mg of corilagin standard in 20 mL of methanol to give a 100 µg/mL solution. | |||||||||||||||
| Chromatographic system |
Detector: UV 270 nm |
|||||||||||||||
| Mobile Phase (gradient mode) |
|
|||||||||||||||
| System suitability requirement |
Perform at least five replicate injections of the corilagin standard solution (100 µg/mL). The requirements of the system suitability parameters are as follow:
|
|||||||||||||||
| Acceptance criteria |
|
PURITY TESTS
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 9% |
| Acid-insoluble ash | Not more than 2% |
| Loss on Drying |
| Not more than 10% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 21% |
| Cold method | Not less than 14% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 13% |
| Cold method | Not less than 7% |
SAFETY TEST
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Aqueous extract of the whole plant has been reported to contain phenolics (e.g. 1-O-galloyl-6-O-luteoyl-α-D-glucose, β-glucogallin, quercetin 3-O-β-D-glucopyranosyl-(2→1)-O-β-D-xylopyranoside and gallic acid), β-sitosterol, acidic arabinogalactan and repandusinic acid A monosodium salt [ 6 , 7 , 8 ].
Aqueous potassium hydroxide extract yielded xylans (e.g. linear β-(1→4)-linked xylan and complex acidic heteroxylan with a (1→4)-linked β-Xylp main chain substituted by rhamnose, arabinose and 4-O-methylglucuronic acid side chains) [ 9 ].
Ethanolic (70%) extract has been found to contain phenolics (e.g. ellagic acid, brevifolin carboxylic acid, ethyl bervifolin carboxylate and niruriflavone), while the hydroalcoholic extract contained quercetin, rutin and gallic acid [ 10 , 11 , 12 ]. The ethanolic (30%) extract had corilagin, gallic acid, geraniin and ellagic acid [ 13 ].
Butanol extract had geraniin, whereas the hexane extract of whole plant contained lignans (e.g. phyllanthin and hypophyllantin), flavonoids (e.g. 8-(3-methyl-but-2-enyl)-2-phenyl chroman-4-one and 2-(4-hydroxyphenyl)-8-(3-methyl-but-2-enyl)-chroman-4-one), long chain hydrocarbons (e.g. triacontanal and tricontanol) and an alkaloid (e.g. ent-norsecurinine) [ 14 , 15 , 16 , 17 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
P. niruri is traditionally used for jaundice, dysentery, dropsy, gonorrhoea, menorrhagia, mild fever, stomach-ache, children’s coughs, diarrhoea, kidney trouble, syphilis, emmenagogue, after a miscarriage and during confinement, sores and used as poultices for skin complaints, including caterpillar itch [ 1 ]. It is also used as diuretic for urinary calculus and for diarrhea, anorexia and hepatitis [ 18 , 19 ].
Biological and pharmacological activities supported by experimental data
Hepatoprotective activity
Aqueous extract of P. niruri young leaves and stem at dose of 50 and 100 mg/kg body weight twice a day for 1 week when administered intraperitoneally and orally to nimesulide-induced hepatic damage in male Swiss Albino mice restored the superoxide dismutase and catalase level and the non-protein thiol glutathione as well as enhance lipid peroxidation [ 20 ].
Fresh young leaves and stem of P. niruri at a dose of 5 mg/kg body weight for 7 days when administered intraperitoneally to nimesulide-induced hepatic damage in male Swiss albino mice has hepatoprotective action against nimesulide-induced oxidative stress due to its antioxidant properties [ 21 ].
Antilithic activity
Aqueous extract of P. niruri whole plant (1.25 mg/mL/day) administered orally to urolithiasis-induced adult male Wistar rats for a duration of 42 days significantly inhibited matrix calculus growth (p < 0.05) and reduced the number of stone satellites (p < 0.05) [ 22 ].
Aqueous extract of P. niruri whole plant (20 µg/g body weight/day) when given orally to adult male Wistar rats for a duration of 50 days has been shown to interfere in the growth and aggregation of calcium oxalate [ 23 ].
Clinical studies
A total of 69 calcium stone forming (CSF) patients which consist of 39 males and 30 females were treated with 450 mg of capsulated P. niruri whereby its water extract was found to reduce urinary calcium in hypercalciuria patients after 3 months treatment [ 24 ].
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Acute toxicity
Oral single dose acute toxicity study on female Sprague Dawley rats (aged between 8 and 12 weeks old) using aqueous mixture of P. niruri whole plant showed no toxic effects on the parameters observed, including behaviours, body weight, food and water intake. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. No-observed-adverse-effect level (NOAEL) is more than 2,000 mg/kg body weight [ 25 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Warning
Discontinue if allergy occurs.
DOSAGE
As decoction: 15-30 g of P. niruri herb in 250 mL of water 2-3 times per day [ 19 ].
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- Burkill IH. A dictionary of the economic products of the Malay Peninsula. Volume 2. London: Crown agents. 1935;p.1718-1719.
- Musa Y, Zaharah A, Wan Zaki WM. 2005. Dukung anak (Phyllanthus amarus). In: Penanaman tumbuhan ubatan & beraroma. (Musa Y, Muhammad Ghawas M, Mansor P. ed.), Serdang: MARDI. 2005;p.14-20.
- Agharkar SP. Medicinal plants of Bombay presidency. Scientific Publisher, Jodhpur, India. 1991.
- Caius JF. The medicinal and poisonous plants of India. Scientific Publisher, Jodhpur India. 1986;p.220–223.
- Gupta OP. Scientific weed management. Today and Tomorrow’s Printers and Publisher, New Delhi. 1984.
- Subeki S, Matsuura H, Takahashi K, Yamasaki M, Yamato O, Maede Y, Katakura K, Kobayashi S, Trimurningsih T, Chairul C, Yoshihara T. Anti-babesial and anti-plasmodial compounds from Phyllanthus niruri. Journal of Natural Products. 2005;68(4):537-539.
- Mellinger CG, Carbonero ER, Noleto GR, Cipriani TR, Oliveira MBM, Gorin PAJ, Iacomini M. Chemical and biological properties of an arabinogalactan from Phyllanthus niruri. Journal of Natural Products. 2005;68(10):1479-1483.
- Ogata T, Higuchi H, Mochida S, Matsumoto H, Kato A, Endo T, Kaji A, Kaji H. HIV-1 reverse transcriptase inhibitor from Phyllanthus niruri. AIDS Research and Human Retroviruses. 1992;8(11):1937-1944.
- Mellinger CG, Carbonero ER, Cipriani TR, Gorin PAJ, Iacomini M. Xylans from the medicinal herb Phyllanthus niruri. Journal of Natural Products. 2005;68(1):129-132.
- Shimizu M, Horie S, Terashima S, Ueno H, Hayashi T, Arisawa M, Suzuki S, Yoshizaki M, Morita N. Studies on aldose reductase inhibitors from natural products II. Active components of a Paraguayan crude drug Para-parai mi, from Phyllanthus niruri. Chemical & Pharmaceutical Bulletin (Tokyo). 1989;37:2531-2532.
- Than NN, Fotso S, Poeggeler B, Hardeland R, Laatsch H. Niruriflavone, a new antioxidant flavone sulfonic acid from Phyllanthus niruri. Zeitschrift für Naturforschung B: A Journal of Chemical Sciences. 2006;61:57-60.
- Boeira VT, Leite CE, Santos AA Jr, Edelweiss MI, Calixto JB, Campos MM, Morrone FB. Effects of the hydroalcoholic extract of Phyllanthus niruri and its isolated compounds on cyclophosphamide-induced hemorrhagic cystitis in mouse. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2011;384(3):265-275.
- Mahdi ES, Noor AM, Sakeena MH, Abdullah GZ, Abdulkarim M, Sattar MA. Identification of phenolic compounds and assessment of in vitro antioxidants activity of 30% ethanolic extracts derived from two Phyllanthus species indigenous to Malaysia. African Journal of Pharmacy and Pharmacology. 2011;5(17);1967-1978.
- Ueno H, Horie S, Nishi Y, Shogawa H, Kawasaki M, Suzuki S, Hayashi T, Arisawa M, Shimizu M, Yoshizaki M, Morita N, Berganza LH, Ferro E, Basualdo I. Chemical and pharmaceutical studies on medicinal plants in paraguay, geraniin, an angiotensin-converting enzyme inhibitor from “Paraparai Mi”, Phyllanthus niruri. Journal of Natural Products. 1988;51(2):357-359.
- Joshi BS, Gawad DH, Pelletier SW, Kartha G, Bandary K. Isolation and structure (X-ray analysis) of ent-norsecurinine, an alkaloid from Phyllanthus niruri. Journal of Natural Products. 1986;49:614-620.
- Symasundar KV, Singh B, Thakur RS, Husain A, Kiso Y, Hikino H. Antihepatotoxic principles of Phyllanthus niruri herbs. Journal of Ethnopharmacology. 1985;14:41-44.
- Shakil NA, Pankaj, Kumar J, Pandey RK, Saxena DB. Nematicidal prenylated flavanones from Phyllanthus niruri. Phytochemistry. 2008;69:759-764.
- Esai Indonesia. Medicinal herbs index in Indonesia. Jakarta. 1986;p.2161.
- Dharma AP. Tanaman obat tradisional Indonesia. Jakarta PN, Balai Pustaka. 1985;p.124.
- Chatterjee M, Sil PC. Hepatoprotective effect of aqueus extract of Phyllanthus niruri on nimesulide-induced oxidative stress in vivo. Indian Journal of Biochemistry & Biophysics. 2006;43(5):299-305.
- Chatterjee M, Sarkar K, Sil PC. Herbal (Phyllanthus niruri) protein isolate protects liver from nimesulide induced oxidative stress. Pathophysiology. 2006;13(2):95-102.
- Freitas AM, Schor N, Boim MA. The effect of Phyllanthus niruri inhibitors of calcium oxalate crystallization and other factor associated with renal stone formation. BJU International. 2002;89(9):829-834.
- Barros ME, Lima R, Mercuri LP, Matos JR, Schor N, Boim MA. Effect of extract of Phyllanthus niruri on crystal deposition in experimental urolithiasis. Urological Research. 2006;34:351–357.
- Nishiura JL, Campos AH, Boim MA, Heilberg IP, Schor N. Phyllanthus niruri normalizes elevated urinary calcium levels in calcium stone forming (CSF) patients. Urological Research. 2004;32(5):362-355.
- Teh BP, Hamzah NF, Rosli SNS, Yahaya MAF, Zakiah I, Murizal Z. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2012. Report No.: HMRC 11-045/01/PN/WP/D.
- Lizuka T. Inhibitory effects of methyl brevifolincarboxylate isolated from Phyllanthus niruri L. on platelet aggregation. Biological and Pharmaceutical Bulletin. 2007;30(2):382-384.