Title
The Use of Neem/ Semambu (Azadirachta indica) Leaves in Coronavirus Disease (COVID-19)
Objective
The objective of this report is to assess current available evidence on the potential of neem (Azadirachta indica) in COVID-19 management based on the following:
- Efficacy: Focus on neem reported properties of 1: antiviral, 2: modulation of immune response, and 3: role as other supportive therapy or management of disease related complications; and their respective potential mechanism(s) of actions.
- Safety of neem
Methodology
Electronic databases were searched using pre-determined terminologies such as ‘azadirachta indica’, ‘antiviral’, immunomodulatory’, ‘immune response’, ‘mechanism of action’, and ‘safety’. All clinical and preclinical studies (both in vitro and in vivo) related to safety and efficacy or effectiveness of A. indica in treating viral diseases were included.
Results and discussion:
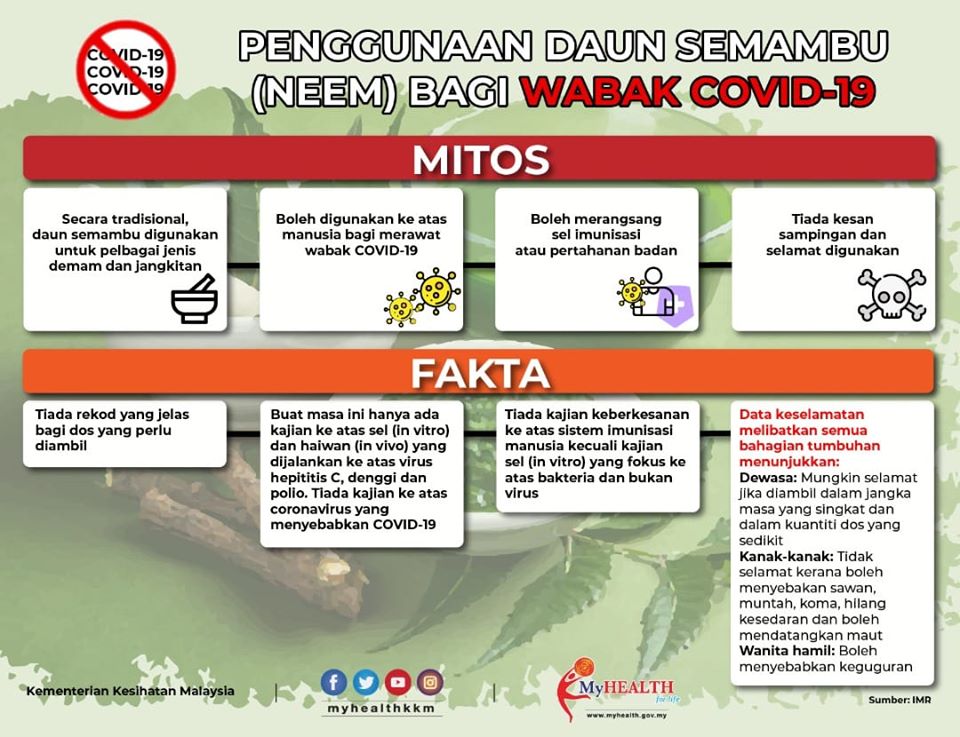
Based on literature search, Azadirachta indica leaves were traditionally used for fever, measles and malaria, both orally and topically, but the amount ingested is not clearly documented. For antiviral effects, there is only one study conducted in humans, small study with 10 patients, while the others were mainly in vitro and in silico simulation docking studies that cover hepatitis C, dengue and polio viruses. As there is no animal study, the possible range of effective doses is not established. From the stimulating aspect of the immune system cells, most of the studies were conducted in vitro on bacteria and not viruses. As for safety, in adults, studies on the plant suggest that oral ingestion might be safe if it is consumed for a short period of time and in small doses but the dose is not well established. Toxicity studies in animal studies have shown high variability with side effects occurring at low doses. Furthermore, ingestion is not safe in children as it can cause fatal neurological reactions; and among pregnant women, which can terminate pregnancy. There is insufficient data to suggest that neem is safe among breastfeeding mothers, hence consumption should be avoided. Animal and in vitro studies also suggested precaution is needed in conditions like diabetes, autoimmune disorders and transplants.
Conclusion
Most activities of neem (Azadirachta indica) are only reported in preclinical study models with insufficient high quality of clinical evidence. Although many phytochemical compounds have been identified from neem, these have not been sufficiently investigated in preclinical and clinical trials to solidly support use as antiviral, especially in COVID-19. The reported adverse reactions, especially in large doses for adults and even in small doses among young children should be considered and weighed against its unclear potential benefits from current available data.
Full report